当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NaNH2 as a Nitrogen Source and Base to Synthesize Triarylamines from Aryl Halides Using Pd-Catalyzed C–N Coupling
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.joc.4c00887 Chinraj Sivarajan, Shriya Saha, Suhel Mulla, Raja Mitra
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.joc.4c00887 Chinraj Sivarajan, Shriya Saha, Suhel Mulla, Raja Mitra
|
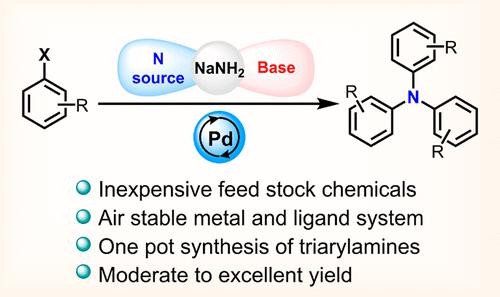
Triarylamines (TAAs) are excellent core structures for multifunctional materials. Reversible single-electron oxidation is the key to versatile applications. Synthesizing these from feedstock materials is inevitable. Here, we report the one-pot synthesis of TAAs from aryl halides and inexpensive NaNH2 as a nitrogen source and base (dual role). The Pd/Xantphos catalytic system shows excellent selectivity toward TAAs from aryl bromides without adding organic amines and an additional base. Various para substituents on the aryl ring show good functional group tolerance in the presence of NaNH2, resulting in moderate to excellent yield (20–91%). Even though the meta-substituted aryl bromides give TAA products in moderate to excellent yields (20–81%), the ortho substitution leads to only diarylamine products. TAAs from aryl chlorides can be achieved only by changing the ligand to Xphos. The mechanistic investigation suggests that three sequential C–N cross-coupling reactions give the TAA products in the presence of NaNH2. The photophysical and electrochemical properties of TAAs and corresponding radicals were tunable based on substitution patterns.
中文翻译:

NaNH2 作为氮源和碱,使用 Pd 催化的 C-N 偶联从芳基卤化物合成三芳胺
三芳胺 (TAA) 是多功能材料的优异核心结构。可逆单电子氧化是实现多种应用的关键。从原料中合成这些是不可避免的。在这里,我们报道了从芳基卤化物和廉价的 NaNH2 作为氮源和碱(双重作用)的一锅法合成 TAA。Pd/Xantphos 催化系统对芳基溴中的 TAA 表现出优异的选择性,无需添加有机胺和额外的碱。在 NaNH2 存在下,芳基环上的各种对位取代基显示出良好的官能团耐受性,从而获得中等至极好的产量 (20–91%)。尽管间位取代的芳基溴得到的 TAA 产物的产率为中等到极高 (20-81%),但邻位取代仅导致二芳胺产物。芳基氯的 TAA 只能通过将配体更改为 Xphos 来实现。机理研究表明,在 NaNH2 存在下,三个连续的 C-N 交叉偶联反应会产生 TAA 产物。TAAs 和相应自由基的光物理和电化学性质可根据取代模式进行调整。
更新日期:2024-11-12
中文翻译:

NaNH2 作为氮源和碱,使用 Pd 催化的 C-N 偶联从芳基卤化物合成三芳胺
三芳胺 (TAA) 是多功能材料的优异核心结构。可逆单电子氧化是实现多种应用的关键。从原料中合成这些是不可避免的。在这里,我们报道了从芳基卤化物和廉价的 NaNH2 作为氮源和碱(双重作用)的一锅法合成 TAA。Pd/Xantphos 催化系统对芳基溴中的 TAA 表现出优异的选择性,无需添加有机胺和额外的碱。在 NaNH2 存在下,芳基环上的各种对位取代基显示出良好的官能团耐受性,从而获得中等至极好的产量 (20–91%)。尽管间位取代的芳基溴得到的 TAA 产物的产率为中等到极高 (20-81%),但邻位取代仅导致二芳胺产物。芳基氯的 TAA 只能通过将配体更改为 Xphos 来实现。机理研究表明,在 NaNH2 存在下,三个连续的 C-N 交叉偶联反应会产生 TAA 产物。TAAs 和相应自由基的光物理和电化学性质可根据取代模式进行调整。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号