当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Iodine-Catalyzed Regioselective C-3 Chalcogenation of 7-Azaindoles: Access to Benzothiophene-Fused 7-Azaindole Analogs
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.joc.4c01237 Krishanu Mondal, Siddhartha Paul, Pallabi Halder, Vishal Talukdar, Parthasarathi Das
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.joc.4c01237 Krishanu Mondal, Siddhartha Paul, Pallabi Halder, Vishal Talukdar, Parthasarathi Das
|
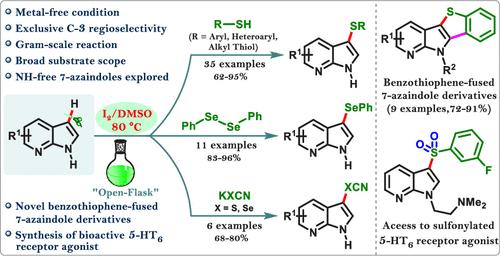
An iodine-catalyzed method has been reported for efficient regioselective C-3 sulfenylation, selenylation, thiocyanation, and selenocyanation of NH-free 7-azaindoles using thiophenols, diselenides, potassium thiocyanates, and selenocyanates, respectively. This approach showcases high efficiency and remarkable versatility, facilitating the synthesis of diverse chalcogenated 7-azaindoles. Additionally, the sulfenylated derivatives have been further diversified to generate a new array of benzothiophene-fused 7-azaindole cores of pharmaceutical interest. The synthetic flexibility of this protocol has been highlighted through the gram-scale synthesis of sulfonylated 7-azaindole-based bioactive 5-HT6 receptor agonists.
中文翻译:

碘催化的 7-氮杂吲哚的区域选择性 C-3 硫属化:苯并噻吩熔合的 7-氮杂吲哚类似物的途径
据报道,一种碘催化方法分别使用硫酚、二硒化物、硫氰酸钾和硒氰酸酯对不含 NH 的 7-氮杂哚进行高效的区域选择性 C-3 次磺酰化、硒化、硫氰化和硒氰化。这种方法显示出高效率和显著的多功能性,有助于合成多种硫属化的 7-氮杂吲哚。此外,次磺酰化衍生物已进一步多样化,以产生新的苯并噻吩熔融 7-氮杂吲哚核心阵列。通过基于磺酰化 7-氮杂吲哚的生物活性 5-HT6 受体激动剂的克级合成,突出了该方案的合成灵活性。
更新日期:2024-11-12
中文翻译:

碘催化的 7-氮杂吲哚的区域选择性 C-3 硫属化:苯并噻吩熔合的 7-氮杂吲哚类似物的途径
据报道,一种碘催化方法分别使用硫酚、二硒化物、硫氰酸钾和硒氰酸酯对不含 NH 的 7-氮杂哚进行高效的区域选择性 C-3 次磺酰化、硒化、硫氰化和硒氰化。这种方法显示出高效率和显著的多功能性,有助于合成多种硫属化的 7-氮杂吲哚。此外,次磺酰化衍生物已进一步多样化,以产生新的苯并噻吩熔融 7-氮杂吲哚核心阵列。通过基于磺酰化 7-氮杂吲哚的生物活性 5-HT6 受体激动剂的克级合成,突出了该方案的合成灵活性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号