当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cycloaddition of Butadiene with Perfluoroethylene: Prediction of a Periselectivity Switch under Pressure
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.joc.4c01361 Mohammed Loukili, Ivan Rivilla, Fernando P. Cossio, Roberto Cammi, Bo Chen
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.joc.4c01361 Mohammed Loukili, Ivan Rivilla, Fernando P. Cossio, Roberto Cammi, Bo Chen
|
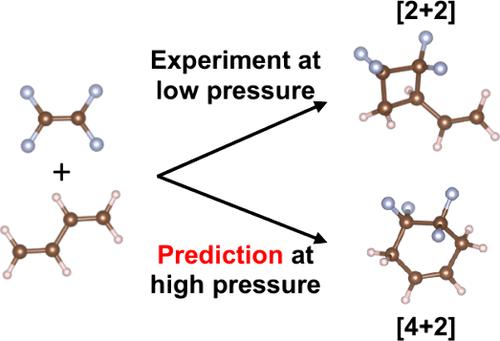
Defying the common Diels–Alder reactivity, the thermal cycloaddition between butadiene and tetrafluoroethylene (TFE) yields exclusively a [2 + 2] cycloadduct via a stepwise diradical mechanism. Here, we study the possibility of reverting to the normal [4 + 2] reactivity in this reaction under high pressure. DFT calculations using the eXtreme Pressure Polarizable Continuum Model (XP-PCM) suggest a more negative activation volume for the concerted [4 + 2] mechanism than the stepwise [2 + 2] mechanism and predict a switch in periselectivity at 1.4 gigapascal (GPa).
中文翻译:

丁二烯与全氟乙烯的环加成反应:压力下 Periselectivity 开关的预测
与常见的 Diels-Alder 反应性相反,丁二烯和四氟乙烯 (TFE) 之间的热环加成反应通过逐步双自由基机制仅产生 [2 + 2] 环加合物。在这里,我们研究了在高压下该反应中恢复到正常 [4 + 2] 反应性的可能性。使用极限压力极化连续体模型 (XP-PCM) 的 DFT 计算表明,协同 [4 + 2] 机制的负激活体积比逐步 [2 + 2] 机制更大,并预测 1.4 吉帕 (GPa) 的周选择性转换。
更新日期:2024-11-11
中文翻译:

丁二烯与全氟乙烯的环加成反应:压力下 Periselectivity 开关的预测
与常见的 Diels-Alder 反应性相反,丁二烯和四氟乙烯 (TFE) 之间的热环加成反应通过逐步双自由基机制仅产生 [2 + 2] 环加合物。在这里,我们研究了在高压下该反应中恢复到正常 [4 + 2] 反应性的可能性。使用极限压力极化连续体模型 (XP-PCM) 的 DFT 计算表明,协同 [4 + 2] 机制的负激活体积比逐步 [2 + 2] 机制更大,并预测 1.4 吉帕 (GPa) 的周选择性转换。

































 京公网安备 11010802027423号
京公网安备 11010802027423号