当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Acid–Solvent Cluster-Promoted General and Regioselective Friedel–Crafts Acylation with Carboxylic Acids
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-10 , DOI: 10.1021/acs.joc.4c02020 Xi Wang, Wanting Fu, Yuanli Ding, Yongcheng An, Liyu Yuan, Jing Tian, Baokun Tang, Zikun Wang
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-10 , DOI: 10.1021/acs.joc.4c02020 Xi Wang, Wanting Fu, Yuanli Ding, Yongcheng An, Liyu Yuan, Jing Tian, Baokun Tang, Zikun Wang
|
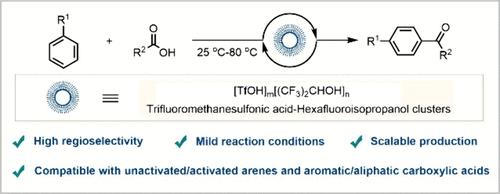
Carboxylic acids are considered to be the most ideal acylating reagents for Friedel–Crafts acylation. However, the low electrophilicity of carboxylic acids and the ability of their byproduct water to deactivate Lewis and Brønsted acids greatly limit their application in this reaction. In this work, we developed a general and regioselective Friedel–Crafts acylation with carboxylic acids, wherein unactivated/activated arenes and various aromatic and aliphatic carboxylic acids were viable starting materials. Key to this accomplishment is the use of trifluoromethanesulfonic acid–hexafluoroisopropanol clusters.
中文翻译:

酸-溶剂簇促进的一般和区域选择性 Friedel-Crafts 酰化与羧酸的酰化
羧酸被认为是 Friedel-Crafts 酰化的最理想酰化试剂。然而,羧酸的低亲电性及其副产物水使路易斯酸和布伦斯特德酸失活的能力极大地限制了它们在该反应中的应用。在这项工作中,我们开发了一种与羧酸的一般和区域选择性 Friedel-Crafts 酰化反应,其中未活化/活化的芳烃和各种芳香族和脂肪族羧酸是可行的起始材料。这一成就的关键是使用三氟甲磺酸-六氟异丙醇簇。
更新日期:2024-11-12
中文翻译:

酸-溶剂簇促进的一般和区域选择性 Friedel-Crafts 酰化与羧酸的酰化
羧酸被认为是 Friedel-Crafts 酰化的最理想酰化试剂。然而,羧酸的低亲电性及其副产物水使路易斯酸和布伦斯特德酸失活的能力极大地限制了它们在该反应中的应用。在这项工作中,我们开发了一种与羧酸的一般和区域选择性 Friedel-Crafts 酰化反应,其中未活化/活化的芳烃和各种芳香族和脂肪族羧酸是可行的起始材料。这一成就的关键是使用三氟甲磺酸-六氟异丙醇簇。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号