当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Metal-Free Synthesis of Benzisoxazolones Utilizing ortho-Ester and ortho-Cyano-Functionalized Diaryliodonium Salts with Protected Hydroxylamines
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-10 , DOI: 10.1021/acs.joc.4c02242 Elghareeb E. Elboray, Taeho Bae, Kotaro Kikushima, Naoko Takenaga, Yasuyuki Kita, Toshifumi Dohi
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-10 , DOI: 10.1021/acs.joc.4c02242 Elghareeb E. Elboray, Taeho Bae, Kotaro Kikushima, Naoko Takenaga, Yasuyuki Kita, Toshifumi Dohi
|
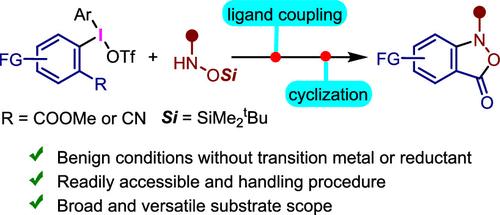
Herein, we report the development of metal-free one/two-pot procedures for the synthesis of benzo[c]isoxazol-3(1H)-one (benzisoxazolone) heterocycles by designing diaryliodonium salts featuring ortho-ester or nitrile functional groups. These react smoothly with protected hydroxylamines under mild conditions to produce N-arylhydroxylamine intermediates, which readily cyclize to give benzisoxazolone derivatives under acidic conditions. This metal-free process maintains the weak N–O bond, tolerates a wide range of diaryliodonium salts and protected hydroxylamines with diverse functional/protecting groups, thereby overcoming the challenges associated with previous transformations. The protocol expands the reaction scope and broadens the chemical space of the fused isoxazolone backbones to include unprecedented five-membered heteroaryl-fused isoxazolones in high yields. This method is also applicable to gram-scale synthesis, and the resulting benzisoxazolones can be effectively derivatized at the N-position to afford valuable compounds.
中文翻译:

利用邻位酯和邻氰基官能化二芳基二芳基铵盐与受保护的羟胺进行苯并异噁唑酮的无金属合成
在此,我们报道了通过设计具有邻酯或腈官能团的二芳基二苯二烯盐合成苯并[c]异噁唑-3(1H)-one(苯并异恶唑酮)杂环的无金属一/双锅程序的开发。它们在温和条件下与受保护的羟胺顺利反应,生成 N-芳基羟胺中间体,在酸性条件下很容易环化得到苯并异恶唑酮衍生物。这种无金属工艺保持了弱的 N-O 键,耐受了多种二芳基二烯铵盐和具有不同官能团/保护基团的受保护羟胺,从而克服了与先前转变相关的挑战。该方案扩大了反应范围并拓宽了熔融异噁唑酮骨架的化学空间,以包括前所未有的高产率五元杂芳基熔融异噁唑酮。该方法也适用于克级合成,所得的苯并异噁唑酮可以在 N 位有效衍生化,从而得到有价值的化合物。
更新日期:2024-11-12
中文翻译:

利用邻位酯和邻氰基官能化二芳基二芳基铵盐与受保护的羟胺进行苯并异噁唑酮的无金属合成
在此,我们报道了通过设计具有邻酯或腈官能团的二芳基二苯二烯盐合成苯并[c]异噁唑-3(1H)-one(苯并异恶唑酮)杂环的无金属一/双锅程序的开发。它们在温和条件下与受保护的羟胺顺利反应,生成 N-芳基羟胺中间体,在酸性条件下很容易环化得到苯并异恶唑酮衍生物。这种无金属工艺保持了弱的 N-O 键,耐受了多种二芳基二烯铵盐和具有不同官能团/保护基团的受保护羟胺,从而克服了与先前转变相关的挑战。该方案扩大了反应范围并拓宽了熔融异噁唑酮骨架的化学空间,以包括前所未有的高产率五元杂芳基熔融异噁唑酮。该方法也适用于克级合成,所得的苯并异噁唑酮可以在 N 位有效衍生化,从而得到有价值的化合物。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号