当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tandem Cyclization of o-Hydroxyphenyl Propargyl Alcohols with Thionucleophiles: A Metal-Free and Modular Access to 3-Fluoroalkylsulfonyl/Thio Benzofurans
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-10 , DOI: 10.1021/acs.joc.4c02338 Lele Wang, Lan Bao, Guili Li, Jinhuan Dong, Xianxiu Xu
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-11-10 , DOI: 10.1021/acs.joc.4c02338 Lele Wang, Lan Bao, Guili Li, Jinhuan Dong, Xianxiu Xu
|
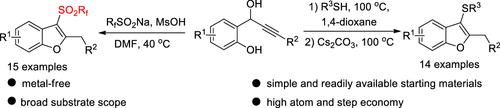
Despite both fluoroalkylsulfonyl groups and benzofurans are important bioactive moieties, the construction of fluoroalkanesulfonylated benzofurans that incorporate these two fragments remains underdeveloped. Here, we report a tandem cyclization protocol to construct a wide range of 3-fluoroalkylsulfonyl benzofurans using readily accessible o-hydroxyphenyl propargyl alcohols and sodium fluoroalkanesulfinates. Furthermore, this synthetic strategy can also be adapted to prepare 3-thio benzofurans by changing the S-nucleophiles to thiols or thiophenols.
中文翻译:

邻羟基苯基丙炔醇与嗜硫粒剂的串联环化反应:3-氟烷基磺酰/硫代苯并呋喃的无金属和模块化可访问
尽管氟烷基磺酰基和苯并呋喃都是重要的生物活性部分,但包含这两个片段的氟烷基磺酰化苯并呋喃的构建仍然不发达。在这里,我们报告了一种串联环化方案,以使用易于获得的邻羟基苯丙基炔基醇和氟烷基亚磺酸钠构建广泛的 3-氟烷基磺酰基苯并呋喃。此外,这种合成策略也可以通过将 S 亲核试剂更改为硫醇或硫酚来适应制备 3-硫代苯并呋喃。
更新日期:2024-11-12
中文翻译:

邻羟基苯基丙炔醇与嗜硫粒剂的串联环化反应:3-氟烷基磺酰/硫代苯并呋喃的无金属和模块化可访问
尽管氟烷基磺酰基和苯并呋喃都是重要的生物活性部分,但包含这两个片段的氟烷基磺酰化苯并呋喃的构建仍然不发达。在这里,我们报告了一种串联环化方案,以使用易于获得的邻羟基苯丙基炔基醇和氟烷基亚磺酸钠构建广泛的 3-氟烷基磺酰基苯并呋喃。此外,这种合成策略也可以通过将 S 亲核试剂更改为硫醇或硫酚来适应制备 3-硫代苯并呋喃。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号