当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Step-Economic Synthesis of Tamsulosin Hydrochloride via Continuous Chlorosulfonation and Biocatalytic Transamination
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.oprd.4c00236 Ágnes Malta-Lakó, Raquel M. Durão, László Poppe, Ricardo F. Mendonça
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.oprd.4c00236 Ágnes Malta-Lakó, Raquel M. Durão, László Poppe, Ricardo F. Mendonça
|
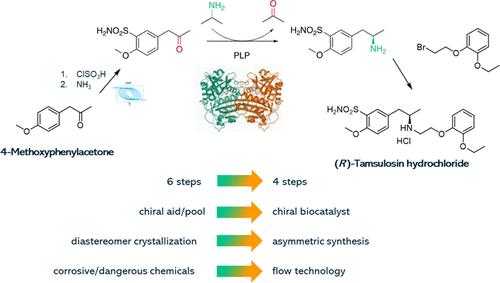
A new process was developed for the synthesis of (R)-tamsulosin in 4 chemical steps from readily available 4-methoxyphenylacetone using continuous chlorosulfonation and biocatalysis. Several conditions were tested for both batch and continuous chlorosulfonation of 4-methoxyphenylacetone. Continuous chlorosulfonation produced a white crystalline solid, while a brown solid or dark oil was consistently obtained when the reaction was performed in batch. Consequently, the sulfonamide intermediate was isolated as a white product in the continuous process, albeit in a slightly lower yield. Immobilized Escherichia coli whole cells overexpressing (R)-selective transaminases from Arthrobacter sp. (ArR-ATA and ArRmut-ATA, natural and engineered, respectively) and Aspergillus terreus (AtR-ATA), along with lyophilized amine transaminase (ATAs), were screened for the key asymmetric synthesis of the chiral amine intermediate. Under optimal conditions, conversions above 90% with >99% enantiomeric excess (ee) were achieved. Furthermore, for process intensification purposes, ATA-412 was covalently immobilized onto surface-activated mesoporous methacrylate beads, achieving quantitative immobilization yields. Immobilization and transamination were scaled up 30-fold, and the synthesized chiral amine intermediate was subjected to N-alkylation without isolation, yielding (R)-tamsulosin hydrochloride. Therefore, after scale-up, this synthesis shows a high potential to replace the current manufacturing process.
中文翻译:

通过连续氯磺化和生物催化转氨反应逐步经济地合成盐酸坦索罗辛
开发了一种使用连续氯磺化和生物催化从现成的 4-甲氧基苯丙酮分 4 个化学步骤合成 (R)-坦索罗辛的新工艺。对 4-甲氧基苯丙酮的批量和连续氯磺化进行了几种条件测试。连续氯磺化生成白色结晶固体,而分批反应时始终获得棕色固体或深色油。因此,磺酰胺中间体在连续工艺中被分离为白色产物,尽管产率略低。筛选了来自节杆菌属 (ArR-ATA 和 ArRmut-ATA,分别为天然和工程化) 和土曲霉 (AtR-ATA) 的过表达 (R) 选择性转氨酶的固定化大肠杆菌全细胞,以及冻干胺转氨酶 (ATA),以寻找手性胺中间体的关键不对称合成。在最佳条件下,>99% 对映体过量 (ee) 实现了高于 90% 的转化率。此外,为了工艺强化目的,将 ATA-412 共价固定在表面活化的介孔甲基丙烯酸酯珠子上,实现定量固定产率。固定化和转氨化放大 30 倍,合成的手性胺中间体未经分离进行 N-烷基化,得到 (R)-盐酸坦索罗辛。因此,在放大生产后,这种合成显示出取代当前制造工艺的巨大潜力。
更新日期:2024-11-11
中文翻译:

通过连续氯磺化和生物催化转氨反应逐步经济地合成盐酸坦索罗辛
开发了一种使用连续氯磺化和生物催化从现成的 4-甲氧基苯丙酮分 4 个化学步骤合成 (R)-坦索罗辛的新工艺。对 4-甲氧基苯丙酮的批量和连续氯磺化进行了几种条件测试。连续氯磺化生成白色结晶固体,而分批反应时始终获得棕色固体或深色油。因此,磺酰胺中间体在连续工艺中被分离为白色产物,尽管产率略低。筛选了来自节杆菌属 (ArR-ATA 和 ArRmut-ATA,分别为天然和工程化) 和土曲霉 (AtR-ATA) 的过表达 (R) 选择性转氨酶的固定化大肠杆菌全细胞,以及冻干胺转氨酶 (ATA),以寻找手性胺中间体的关键不对称合成。在最佳条件下,>99% 对映体过量 (ee) 实现了高于 90% 的转化率。此外,为了工艺强化目的,将 ATA-412 共价固定在表面活化的介孔甲基丙烯酸酯珠子上,实现定量固定产率。固定化和转氨化放大 30 倍,合成的手性胺中间体未经分离进行 N-烷基化,得到 (R)-盐酸坦索罗辛。因此,在放大生产后,这种合成显示出取代当前制造工艺的巨大潜力。

































 京公网安备 11010802027423号
京公网安备 11010802027423号