当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cu(I)-Catalyzed C(sp3)–H Functionalization of Amino Acids with Benzimidate and Reactive Oxygen Species (ROS) To Synthesize Triazines and 2-Pyrrolidinones
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03536 Subhasis Pal, Rajesh Nandi, Anindya S. Manna, Debanjana Bag, Rajjakfur Rahaman, Dilip K. Maiti
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03536 Subhasis Pal, Rajesh Nandi, Anindya S. Manna, Debanjana Bag, Rajjakfur Rahaman, Dilip K. Maiti
|
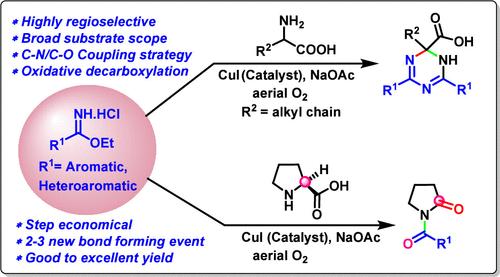
An easily accessible Cu(I)-catalyzed regioselective oxidative C–N/C–O cross-coupling organic transformation has been disclosed for the syntheses of variably functionalized triazines and N-benzoylpyrrolidin-2-ones through the involvement of C(sp3)–H bond functionalization, which is unknown in the literature. This general synthetic method is extended for decarboxylative oxidation of amino acids to install carbonyl functionality. It facilitates the formation of 2–3 new bonds through the cross-coupling strategy involving benzimidates, amino acids, and in situ-generated reactive oxygen species (ROS) from the aerial O2 as the sole oxidant. The key utilities of the new reactions are demonstrated by its operational simplicity, regioselectivity, robustness, and broad substrate scope with high yields.
中文翻译:

Cu(I) 催化的 C(sp3)-H 氨基酸与苯并咪酯和活性氧 (ROS) 官能化合成三嗪和 2-吡咯烷酮
通过参与 C(sp3)-H 键官能化,已经发现了一种易于接近的 Cu(I) 催化的区域选择性氧化 C-N/C-O 交叉偶联有机转化,用于合成可变官能化的三嗪和 N-苯并酰吡咯烷-2-酮,这在文献中是未知的。这种通用的合成方法扩展到氨基酸的脱羧氧化以建立羰基官能团。它通过涉及苯并胺酸盐、氨基酸和作为唯一氧化剂的空气 O2 原位生成的活性氧 (ROS) 的交叉偶联策略促进 2-3 个新键的形成。新反应的关键用途体现在其操作简单性、区域选择性、稳定性和广泛的底物范围和高产量上。
更新日期:2024-11-12
中文翻译:

Cu(I) 催化的 C(sp3)-H 氨基酸与苯并咪酯和活性氧 (ROS) 官能化合成三嗪和 2-吡咯烷酮
通过参与 C(sp3)-H 键官能化,已经发现了一种易于接近的 Cu(I) 催化的区域选择性氧化 C-N/C-O 交叉偶联有机转化,用于合成可变官能化的三嗪和 N-苯并酰吡咯烷-2-酮,这在文献中是未知的。这种通用的合成方法扩展到氨基酸的脱羧氧化以建立羰基官能团。它通过涉及苯并胺酸盐、氨基酸和作为唯一氧化剂的空气 O2 原位生成的活性氧 (ROS) 的交叉偶联策略促进 2-3 个新键的形成。新反应的关键用途体现在其操作简单性、区域选择性、稳定性和广泛的底物范围和高产量上。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号