当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of Polycyclic Heterocyclic Compounds via Friedel–Crafts Reaction/Cyclization Reaction Catalyzed by Nickel Catalyst
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03674 Shujun Tong, Zhifei Zhao, Liangjian Hu, Shi-Wu Li
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03674 Shujun Tong, Zhifei Zhao, Liangjian Hu, Shi-Wu Li
|
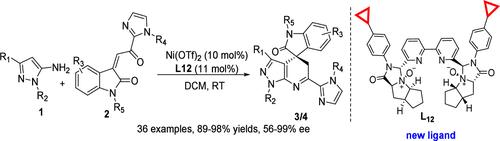
The first highly enantioselective asymmetric Friedel–Crafts reaction/cyclization reaction of 5-aminopyrazoles with 3-alkenyloxindoles to afford polycyclic heterocyclic compounds bearing an all-carbon quaternary stereocenter catalyzed by a complex of NiII with the C2-symmetric bipyridine-N,N′-dioxide ligand L12 has been developed. The relevant products with a wide range of substrates and good functional tolerance were obtained in 89–98% yield with 56–99% ee in the presence of 10 mol % Ni(OTf)2 and 11 mol % L12 in DCM at 25 °C. Moreover, an experiment on scaling up the process along with the transformations of the cycloadducts further emphasized the practical application of the synthesis.
中文翻译:

通过镍催化剂催化的 Friedel-Crafts 反应/环化反应不对称合成多环杂环化合物
5-氨基吡唑与 3-烯基氧吲哚的第一个高度对映选择性不对称 Friedel-Crafts 反应/环化反应,得到带有全碳四元立体中心的多环杂环化合物,该化合物由 NiII 与 C2-对称联吡啶-N,N′-二氧化物配体 L 的络合物催化 12已经开发出来。在 25 °C 下,在 10 mol % Ni(OTf)2 和 11 mol % L12 in DCM 中存在下,以 89-98% 的收率和 56-99% 的 ee 获得具有广泛底物和功能耐受性的相关产品。 此外,一项关于扩大该过程以及环加合物转化的实验进一步强调了合成的实际应用。
更新日期:2024-11-11
中文翻译:

通过镍催化剂催化的 Friedel-Crafts 反应/环化反应不对称合成多环杂环化合物
5-氨基吡唑与 3-烯基氧吲哚的第一个高度对映选择性不对称 Friedel-Crafts 反应/环化反应,得到带有全碳四元立体中心的多环杂环化合物,该化合物由 NiII 与 C2-对称联吡啶-N,N′-二氧化物配体 L 的络合物催化 12已经开发出来。在 25 °C 下,在 10 mol % Ni(OTf)2 和 11 mol % L12 in DCM 中存在下,以 89-98% 的收率和 56-99% 的 ee 获得具有广泛底物和功能耐受性的相关产品。 此外,一项关于扩大该过程以及环加合物转化的实验进一步强调了合成的实际应用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号