当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Biaryl N-Heterocyclic Carbene–Palladium Catalysts with Anagostic C–H···Pd Interaction for Enantioselective Desymmetric C–N Cross-Coupling
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03709 Woosong Han, Huijeong Ryu, Changmuk Kang, Sukwon Hong
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03709 Woosong Han, Huijeong Ryu, Changmuk Kang, Sukwon Hong
|
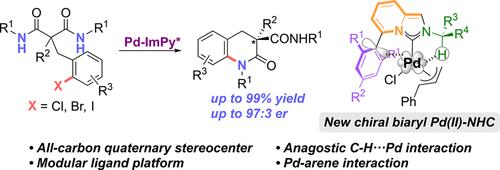
Novel chiral biaryl imidazo[1,5-a]pyridine carbene–palladium complexes (ImPy–Pd) featuring an anagostic C–H···Pd interaction and a C5-aryl substituent have been developed and successfully applied to the Pd-catalyzed enantioselective desymmetric C–N cross-coupling of malonamide derivatives, providing chiral 3,4-dihydroquinoline-2-ones with quaternary stereocenters in high yields (≤99%) and enantioselectivities (≤97:3 er). The chiral catalyst exerts stereocontrol by restricting the rotation of substituents around the metal center through anagostic interactions with sterically bulky substituents.
中文翻译:

手性联芳基 N-杂环卡宾-钯催化剂与抗作用 C–H···对映选择性不对称 C-N 交叉偶联的 Pd 相互作用
新型手性联芳基咪唑[1,5-a]吡啶卡宾-钯配合物(ImPy-Pd),具有模拟的C-H···Pd 相互作用和 C5-芳基取代基已被开发并成功应用于丙二酰胺衍生物的 Pd 催化的对映选择性不对称 C-N 交叉偶联,以高产率 (≤99%) 和对映选择性 (≤97:3 er) 提供具有四元立体中心的手性 3,4-二氢喹啉-2-酮。手性催化剂通过与空间大体积取代基的模拟相互作用来限制取代基围绕金属中心的旋转,从而发挥立体控制。
更新日期:2024-11-12
中文翻译:

手性联芳基 N-杂环卡宾-钯催化剂与抗作用 C–H···对映选择性不对称 C-N 交叉偶联的 Pd 相互作用
新型手性联芳基咪唑[1,5-a]吡啶卡宾-钯配合物(ImPy-Pd),具有模拟的C-H···Pd 相互作用和 C5-芳基取代基已被开发并成功应用于丙二酰胺衍生物的 Pd 催化的对映选择性不对称 C-N 交叉偶联,以高产率 (≤99%) 和对映选择性 (≤97:3 er) 提供具有四元立体中心的手性 3,4-二氢喹啉-2-酮。手性催化剂通过与空间大体积取代基的模拟相互作用来限制取代基围绕金属中心的旋转,从而发挥立体控制。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号