当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemodivergent Reactions of Aromatic Ring-Annulated Hexahydrocyclopentafurans with Various Aldehydes
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03734 Tinatina Junior Kindala, Kaede Yano, Kazuhiko Takatori, Megumi Mizukami, Shinji Nagumo
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03734 Tinatina Junior Kindala, Kaede Yano, Kazuhiko Takatori, Megumi Mizukami, Shinji Nagumo
|
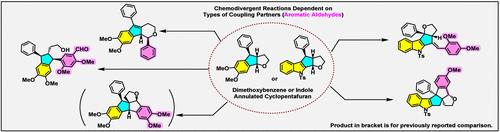
Hexahydro-2H-cyclopenta[b]furan fused to electron-rich aromatic rings reacts with various aromatic aldehydes in different modes to build diverse frameworks. The reaction of a dimethoxybenzene-fused cyclopentafuran generated diquinanes fused with a hydrofuran ring, indenopyrans, or diarylindanes depending upon the type of aromatic aldehydes, whereas an indole-annulated cyclopentafuran generated another type of diquinane fused with hydrofuran ring or benzylidenecyclopentafuran. The chemodivergence is due to the different properties between indole- and dimethoxybenzene-fused hydrocyclopentafurans. Namely, Lewis acid-promoted furan-ring opening of the substrates resulted in the formation of an electrophilic or a nucleophilic intermediate, respectively. Additionally, the observed chemodivergence can be attributed to the distinctive electronic properties of three classified aromatic aldehydes. Of particular interest is that 2,4-dimethoxybenzaldehyde reacted with the dimethoxybenzene-fused cyclopentafuran at the benzene ring, whereas it reacted with the indole-fused cyclopentafuran at the formyl group.
中文翻译:

芳香环环化六氢环戊呋喃与各种醛类的化学分歧反应
六氢-2H-环戊[b]呋喃与富电子芳香环融合,与各种芳香醛以不同模式反应,构建多种骨架。二甲氧基苯熔融的环戊呋喃反应生成与氢呋喃环熔融的二呋喃烷、茚并吡喃或二芳基呋喃,具体取决于芳香醛的类型,而吲哚环状呋喃生成另一种类型的二呋喃与氢呋喃环或亚苄基环戊呋喃熔合。化学分歧是由于吲哚和二甲氧基苯稠合氢环戊呋喃之间的性质不同。即,Lewis 酸促进底物的呋喃环打开分别导致亲电或亲核中间体的形成。此外,观察到的化学分歧可归因于三种分类芳香醛的独特电子性质。特别有趣的是,2,4-二甲氧基苯甲醛在苯环处与二甲氧基苯熔融的环戊呋喃反应,而在甲酰基处与吲哚熔融的环戊呋喃反应。
更新日期:2024-11-12
中文翻译:

芳香环环化六氢环戊呋喃与各种醛类的化学分歧反应
六氢-2H-环戊[b]呋喃与富电子芳香环融合,与各种芳香醛以不同模式反应,构建多种骨架。二甲氧基苯熔融的环戊呋喃反应生成与氢呋喃环熔融的二呋喃烷、茚并吡喃或二芳基呋喃,具体取决于芳香醛的类型,而吲哚环状呋喃生成另一种类型的二呋喃与氢呋喃环或亚苄基环戊呋喃熔合。化学分歧是由于吲哚和二甲氧基苯稠合氢环戊呋喃之间的性质不同。即,Lewis 酸促进底物的呋喃环打开分别导致亲电或亲核中间体的形成。此外,观察到的化学分歧可归因于三种分类芳香醛的独特电子性质。特别有趣的是,2,4-二甲氧基苯甲醛在苯环处与二甲氧基苯熔融的环戊呋喃反应,而在甲酰基处与吲哚熔融的环戊呋喃反应。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号