当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Precisive Incorporation of Multiple Nitrogen Sources into Benzoxazoles via an Iodine-Mediated Electrochemical Four-Component Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03844 Tong Li, Kai Li, Jiajia Yu, Qi Sun, Zhiyong Wang
Organic Letters ( IF 4.9 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.orglett.4c03844 Tong Li, Kai Li, Jiajia Yu, Qi Sun, Zhiyong Wang
|
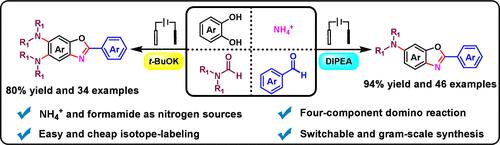
An iodine-mediated electrochemical four-component reaction was developed to construct aromatic C–N bonds by making use of a simple nitrogen source, such as NH4+ and formamide. By virtue of this reaction, a variety of benzoxazoles bearing different substituents can be selectively modulated by using different bases. This protocol features a broad substrate scope and good scalability, is transition metal-free and chemical oxidant-free, and exhibits controlled product distribution. Additionally, it also enables a versatile platform for various isotope-labeled (15N and CD3) benzoxazoles.
中文翻译:

通过碘介导的电化学四组分反应将多个氮源精确掺入苯并恶唑中
开发了一种碘介导的电化学四组分反应,利用简单的氮源(如 NH4+ 和甲酰胺)构建芳香族 C-N 键。通过该反应,可以使用不同的碱选择性地调节各种带有不同取代基的苯并恶唑。该协议具有广泛的底物范围和良好的可扩展性,不含过渡金属和化学氧化剂,并表现出受控的产品分布。此外,它还为各种同位素标记(15N 和 CD3)苯并恶唑提供了一个多功能平台。
更新日期:2024-11-11
中文翻译:

通过碘介导的电化学四组分反应将多个氮源精确掺入苯并恶唑中
开发了一种碘介导的电化学四组分反应,利用简单的氮源(如 NH4+ 和甲酰胺)构建芳香族 C-N 键。通过该反应,可以使用不同的碱选择性地调节各种带有不同取代基的苯并恶唑。该协议具有广泛的底物范围和良好的可扩展性,不含过渡金属和化学氧化剂,并表现出受控的产品分布。此外,它还为各种同位素标记(15N 和 CD3)苯并恶唑提供了一个多功能平台。































 京公网安备 11010802027423号
京公网安备 11010802027423号