当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predicting the Solubility of Amino Acids and Peptides with the SAFT-γ Mie Approach: Neutral and Charged Models
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.iecr.4c02995 Ahmed Alyazidi, Shubhani Paliwal, Felipe A. Perdomo, Amy Mead, Mingxia Guo, Jerry Y. Y. Heng, Thomas Bernet, Andrew J. Haslam, Claire S. Adjiman, George Jackson, Amparo Galindo
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-11 , DOI: 10.1021/acs.iecr.4c02995 Ahmed Alyazidi, Shubhani Paliwal, Felipe A. Perdomo, Amy Mead, Mingxia Guo, Jerry Y. Y. Heng, Thomas Bernet, Andrew J. Haslam, Claire S. Adjiman, George Jackson, Amparo Galindo
|
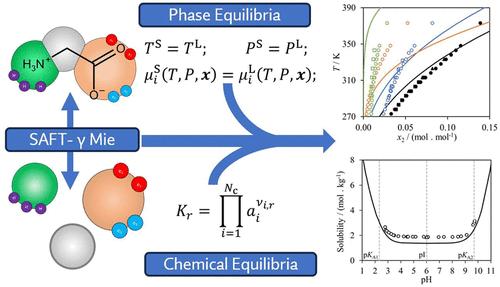
Modeling approaches that can be used to predict accurately the solubility of amino acids and peptides are of interest for the design of new pharmaceutical processes and in the development of new peptide-based therapeutics. We investigate the capability of the SAFT-γ Mie group-contribution approach to predict the aqueous and alcohol solubility of glycine, alananine, valine, leucine, and serine and of di- and tripeptides containing these amino acids. New SAFT-γ Mie group interactions are characterized using experimental thermodynamic and phase-equilibrium data of compounds and mixtures that contain groups relevant to the amino acids and peptides, but no solubility data (except for the case of glycine). Once all the group interaction parameters are developed, predictive solid–liquid solubility calculations are carried out. Neutral and charged models are considered to account explicitly for the zwitterionic nature of the molecules in aqueous solution, and the solubility of the solution is presented as a function of pH. A detailed discussion of the molecular models and Helmholtz free-energy expressions used to represent the ionic and zwitterionic forms of the amino acids, together with their speciation in solution is also provided. Overall, very good agreement with available data is shown, with an absolute average deviation (AAD) in mole fraction of 0.0038 over 283 solubility data points for the amino acids studied and an AAD in mole fraction of 0.02128 over 141 peptide-solubility points when the systems are studied at their isoelectric point and neutral models are used. The solubility as a function of pH for a range of temperatures is also predicted accurately when charged models are incorporated. These results confirm the predictive accuracy of the SAFT-γ Mie method and pave the way for future studies involving larger peptides.
中文翻译:

使用 SAFT-γ Mie 方法预测氨基酸和肽的溶解度:中性和带电模型
可用于准确预测氨基酸和肽溶解度的建模方法对于设计新的制药工艺和开发新的基于肽的疗法具有重要意义。我们研究了 SAFT-γ Mie 群贡献方法预测甘氨酸、丙氨酸、缬氨酸、亮氨酸和丝氨酸以及含有这些氨基酸的二肽和三肽的水溶性和醇溶性的能力。新的 SAFT-γ Mie 基团相互作用使用化合物和混合物的实验热力学和相平衡数据来表征,这些数据包含与氨基酸和肽相关的基团,但没有溶解度数据(甘氨酸的情况除外)。一旦开发了所有组相互作用参数,就可以进行预测性固液溶解度计算。中性和带电模型被认为明确解释了分子在水溶液中的两性离子性质,溶液的溶解度表示为 pH 值的函数。还详细讨论了用于表示氨基酸的离子和两性离子形式的分子模型和亥姆霍兹自由能表达式,以及它们在溶液中的形态。总体而言,与现有数据显示出非常好的一致性,当在等电点研究系统并使用中性模型时,所研究氨基酸在 283 个溶解度数据点上的摩尔分数绝对平均偏差 (AAD) 为 0.0038,在 141 个肽溶解度点上的摩尔分数为 0.02128。当结合带电模型时,还可以准确预测一定温度范围内溶解度随 pH 值的变化。 这些结果证实了 SAFT-γ Mie 方法的预测准确性,并为未来涉及更大肽的研究铺平了道路。
更新日期:2024-11-11
中文翻译:

使用 SAFT-γ Mie 方法预测氨基酸和肽的溶解度:中性和带电模型
可用于准确预测氨基酸和肽溶解度的建模方法对于设计新的制药工艺和开发新的基于肽的疗法具有重要意义。我们研究了 SAFT-γ Mie 群贡献方法预测甘氨酸、丙氨酸、缬氨酸、亮氨酸和丝氨酸以及含有这些氨基酸的二肽和三肽的水溶性和醇溶性的能力。新的 SAFT-γ Mie 基团相互作用使用化合物和混合物的实验热力学和相平衡数据来表征,这些数据包含与氨基酸和肽相关的基团,但没有溶解度数据(甘氨酸的情况除外)。一旦开发了所有组相互作用参数,就可以进行预测性固液溶解度计算。中性和带电模型被认为明确解释了分子在水溶液中的两性离子性质,溶液的溶解度表示为 pH 值的函数。还详细讨论了用于表示氨基酸的离子和两性离子形式的分子模型和亥姆霍兹自由能表达式,以及它们在溶液中的形态。总体而言,与现有数据显示出非常好的一致性,当在等电点研究系统并使用中性模型时,所研究氨基酸在 283 个溶解度数据点上的摩尔分数绝对平均偏差 (AAD) 为 0.0038,在 141 个肽溶解度点上的摩尔分数为 0.02128。当结合带电模型时,还可以准确预测一定温度范围内溶解度随 pH 值的变化。 这些结果证实了 SAFT-γ Mie 方法的预测准确性,并为未来涉及更大肽的研究铺平了道路。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号