当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fe-Doped Ni-Based Catalysts Surpass Ir-Baselines for Oxygen Evolution Due to Optimal Charge-Transfer Characteristics
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-11 , DOI: 10.1021/acscatal.4c04489 Mai-Anh Ha, Shaun M. Alia, Andrew G. Norman, Elisa M. Miller
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-11 , DOI: 10.1021/acscatal.4c04489 Mai-Anh Ha, Shaun M. Alia, Andrew G. Norman, Elisa M. Miller
|
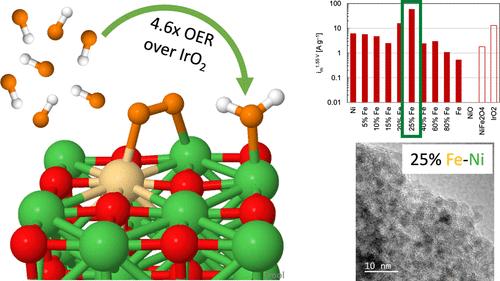
Ni-based catalysts with Co or Fe can potentially replace precious Ir-based catalysts for the rate-limiting oxygen evolution reaction (OER) in anion-exchange membrane (AEM) electrolyzers. In this study, density functional theory (DFT) calculations provide atomic- and electronic-level resolution on how the inclusion of Co or Fe can overcome the inactivity of NiO catalysts and even enable them to surpass IrO2 in activating key steps to the OER. Namely, NiO resists binding the key OH* intermediate and presents a high energetic barrier to forming the O*. Co- and Fe-substitution of Ni active sites allows for the stronger binding of OH* and preferentially activates O*/O2* formation, with Fe-substitution increasing the OER activity substantially as compared to Co-substitution. Whereas IrO2 requires an activation energy of 0.34–0.49 eV to form O2, this step is spontaneous on Fesub-NiO. Electrodeposition of polycrystalline electrodes and synthesized nanoparticles exploit the Co or Fe presence, with Fe particularly exhibiting greater activity: Tafel slopes indicate a significant change in the mechanism as compared to pure NiO, validating the theoretical predictions of OER activation at different steps. High-performing synthesized nanoparticles of 25% Fe–Ni exhibited a 4.6 times improvement over IrO2 and a 34% improvement over RuO2, showcasing that non-platinum group metal catalysts can outperform platinum group metals. High-resolution transmission electron microscopy further highlights the advantages of Fe–Ni oxide synthesized nanoparticles over commercial catalysts: small, randomly oriented nanoparticles expose greater edge sites than large nanoparticles typical of commercially available materials.
中文翻译:

由于具有最佳的电荷转移特性,Fe 掺杂 Ni 基催化剂的析氧性能超过了 Ir 基线
在阴离子交换膜 (AEM) 电解槽中,含 Co 或 Fe 的镍基催化剂有可能取代珍贵的 Ir 基催化剂,用于限速析氧反应 (OER)。在这项研究中,密度泛函理论 (DFT) 计算提供了原子和电子级分辨率,说明包含 Co 或 Fe 如何克服 NiO 催化剂的无活性,甚至使它们在激活 OER 的关键步骤方面超过 IrO2。也就是说,NiO 抵抗与关键 OH* 中间体的结合,并为形成 O* 提供了高能屏障。Ni 活性位点的 Co- 和 Fe 取代允许 OH* 更强的结合,并优先激活 O*/O2* 的形成,与共取代相比,Fe 取代显着增加了 OER 活性。IrO2 需要 0.34-0.49 eV 的活化能才能形成 O2,而这一步在 Fe亚 NiO 上是自发的。多晶电极和合成纳米颗粒的电沉积利用了 Co 或 Fe 的存在,其中 Fe 尤其表现出更大的活性:与纯 NiO 相比,塔菲尔斜率表明机制发生了显着变化,验证了 OER 在不同步骤激活的理论预测。25% Fe-Ni 的高性能合成纳米颗粒比 IrO2 提高了 4.6 倍,比 RuO2 提高了 34%,这表明非铂族金属催化剂的性能优于铂族金属。高分辨率透射电子显微镜进一步突出了 Fe-Ni 氧化物合成纳米颗粒相对于商业催化剂的优势:小的、随机取向的纳米颗粒比市售材料的典型大纳米颗粒暴露出更大的边缘位点。
更新日期:2024-11-12
中文翻译:

由于具有最佳的电荷转移特性,Fe 掺杂 Ni 基催化剂的析氧性能超过了 Ir 基线
在阴离子交换膜 (AEM) 电解槽中,含 Co 或 Fe 的镍基催化剂有可能取代珍贵的 Ir 基催化剂,用于限速析氧反应 (OER)。在这项研究中,密度泛函理论 (DFT) 计算提供了原子和电子级分辨率,说明包含 Co 或 Fe 如何克服 NiO 催化剂的无活性,甚至使它们在激活 OER 的关键步骤方面超过 IrO2。也就是说,NiO 抵抗与关键 OH* 中间体的结合,并为形成 O* 提供了高能屏障。Ni 活性位点的 Co- 和 Fe 取代允许 OH* 更强的结合,并优先激活 O*/O2* 的形成,与共取代相比,Fe 取代显着增加了 OER 活性。IrO2 需要 0.34-0.49 eV 的活化能才能形成 O2,而这一步在 Fe亚 NiO 上是自发的。多晶电极和合成纳米颗粒的电沉积利用了 Co 或 Fe 的存在,其中 Fe 尤其表现出更大的活性:与纯 NiO 相比,塔菲尔斜率表明机制发生了显着变化,验证了 OER 在不同步骤激活的理论预测。25% Fe-Ni 的高性能合成纳米颗粒比 IrO2 提高了 4.6 倍,比 RuO2 提高了 34%,这表明非铂族金属催化剂的性能优于铂族金属。高分辨率透射电子显微镜进一步突出了 Fe-Ni 氧化物合成纳米颗粒相对于商业催化剂的优势:小的、随机取向的纳米颗粒比市售材料的典型大纳米颗粒暴露出更大的边缘位点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号