当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intermolecular hydrogen bonding delineates the stability of non-canonical adenine base pairs: a first-principles study
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-11 , DOI: 10.1039/d4cp02875a Nicholas Adu-Effah, Nabanita Saikia
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-11 , DOI: 10.1039/d4cp02875a Nicholas Adu-Effah, Nabanita Saikia
|
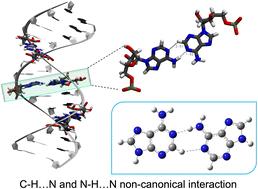
Non-canonical nucleobase pairs differ from canonical Watson–Crick (WC) pairs in their hydrogen bonding patterns. This study uses density functional theory with empirical dispersion correction to examine the stability and electronic properties of free adenine dimers stabilized by hydrogen bonds along the WC, Sugar (S), and Hoogsteen (H) edges. Dispersion correction is crucial for accurate interaction energy evaluation. The most stable adenine dimer is stabilized by N–H⋯N hydrogen bonds in gas and solvent phases. Binding energy decreases by ∼10.2 kcal mol−1 for dimers with both C–H⋯N and N–H⋯N bonds, increasing the donor–acceptor distance. However, with a sugar–phosphate backbone, dimers with C–H⋯N and N–H⋯N bonds have higher binding energy in an implicit solvent, emphasizing the role of C–H⋯N interactions in stability and nucleic acid folding dynamics. This study highlights noncovalent interactions, such as hydrogen bonding and π–π stacking, within adenine pairs with potential applications in biosensing and DNA-based self-assembly on nanomaterial interfaces.
中文翻译:

分子间氢键描绘了非经典腺嘌呤碱基对的稳定性:第一性原理研究
非经典核碱基对与经典 Watson-Crick (WC) 对的氢键模式不同。本研究使用密度泛函理论和经验色散校正来检查沿 WC、Sugar (S) 和 Hoogsteen (H) 边缘的氢键稳定的游离腺嘌呤二聚体的稳定性和电子性质。色散校正对于准确的相互作用能评估至关重要。最稳定的腺嘌呤二聚体在气相和溶剂相中被 N-H⋯N 氢键稳定。对于同时具有 C-H⋯N 和 N-H⋯N 键的二聚体,结合能降低 ∼10.2 kcal mol-1,从而增加供体-受体距离。然而,在糖-磷酸骨架下,具有 C-H⋯N 和 N-H⋯N 键的二聚体在隐含溶剂中具有更高的结合能,强调了 C-H⋯N 相互作用在稳定性和核酸折叠动力学中的作用。本研究强调了腺嘌呤对内的非共价相互作用,例如氢键和 π-π 堆叠,以及在纳米材料界面上基于 DNA 的自组装中的生物传感和潜在应用。
更新日期:2024-11-11
中文翻译:

分子间氢键描绘了非经典腺嘌呤碱基对的稳定性:第一性原理研究
非经典核碱基对与经典 Watson-Crick (WC) 对的氢键模式不同。本研究使用密度泛函理论和经验色散校正来检查沿 WC、Sugar (S) 和 Hoogsteen (H) 边缘的氢键稳定的游离腺嘌呤二聚体的稳定性和电子性质。色散校正对于准确的相互作用能评估至关重要。最稳定的腺嘌呤二聚体在气相和溶剂相中被 N-H⋯N 氢键稳定。对于同时具有 C-H⋯N 和 N-H⋯N 键的二聚体,结合能降低 ∼10.2 kcal mol-1,从而增加供体-受体距离。然而,在糖-磷酸骨架下,具有 C-H⋯N 和 N-H⋯N 键的二聚体在隐含溶剂中具有更高的结合能,强调了 C-H⋯N 相互作用在稳定性和核酸折叠动力学中的作用。本研究强调了腺嘌呤对内的非共价相互作用,例如氢键和 π-π 堆叠,以及在纳米材料界面上基于 DNA 的自组装中的生物传感和潜在应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号