当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
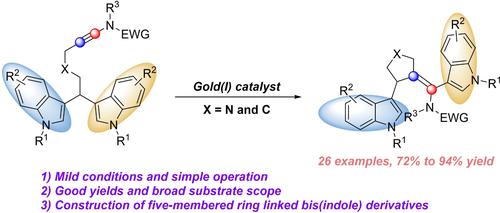
Gold(I)-Catalyzed Regioselective Cycloisomerization of Bis(Indol-3-Yl)-Ynamides to Access Five-Membered Ring Linked Bisindole Derivatives
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-11-10 , DOI: 10.1002/adsc.202401052 Zhanshuai Xiao, Yin Wei, Min Shi
中文翻译:

金(I) 催化的双(吲哚-3-基)-ynami 的区域选择性环异构化以获得五元环连接的双辛哚衍生物
更新日期:2024-11-10
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-11-10 , DOI: 10.1002/adsc.202401052 Zhanshuai Xiao, Yin Wei, Min Shi
|
A gold(I)-catalyzed regioselective cycloisomerization of bis(indol-3-yl)-ynamides for the rapid construction of five-membered ring linked bisindole derivatives has been reported, affording the desired products in the range of 72%–94% yields under mild conditions along with broad substrate scope. Moreover, DFT calculation of the NBO (natural bond orbital) charge supports the origin of its regioselectivity.
中文翻译:

金(I) 催化的双(吲哚-3-基)-ynami 的区域选择性环异构化以获得五元环连接的双辛哚衍生物
据报道,金 (I) 催化的双(吲哚-3-基)-ynami 区域选择性环异构化用于快速构建五元环连接的双吲哚衍生物,在温和条件下提供 72%–94% 产率范围内的理想产物以及广泛的底物范围。此外,NBO(天然键轨道)电荷的 DFT 计算支持其区域选择性的来源。































 京公网安备 11010802027423号
京公网安备 11010802027423号