Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational descriptor for electrochemical currents of carbon dioxide reduction on Cu facets
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-10 , DOI: 10.1016/j.jcat.2024.115836 Timothy T. Yang, Wissam A. Saidi
Journal of Catalysis ( IF 6.5 ) Pub Date : 2024-11-10 , DOI: 10.1016/j.jcat.2024.115836 Timothy T. Yang, Wissam A. Saidi
|
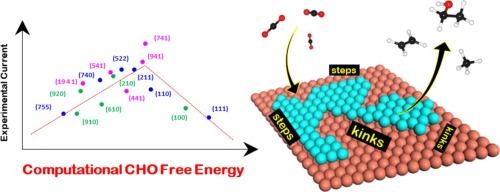
Computation screening is crucial for designing efficient electrochemical catalysts for carbon dioxide (CO2 R) reduction that produce valuable hydrocarbons and oxygenates. Herein, leveraging density functional theory calculations for the CO adsorption energy Δ E C O Δ E C O 2 R electrochemical currents (ACS Catal. 2022, 12, 11, 6578–6588). Examining ab initio thermodynamics of early critical intermediates CO*, COH*, and CHO*, we find that CO* → Beyond the general CO adsorption energy that only shows a linear trend with CO2 R activity, we show that the reaction free energy of CO* → is the descriptor for the overall CO2 R activity for Cu facets, as it displays a volcano relationship with the experimental current. Importantly, we show that high step and kink density of the Cu terminations not only enhances CO adsorption strength but also modulates the CO* →
中文翻译:

Cu 刻面上二氧化碳还原的电化学电流的计算描述符
计算筛选对于设计用于二氧化碳 (CO2R) 还原的高效电化学催化剂至关重要,这些催化剂可产生有价值的碳氢化合物和含氧化合物。在此,利用 17 个 Cu 终端上 CO 吸附能 ΔECO 的密度泛函理论计算,我们发现 ΔECO 与实验测量的 CO2R 电化学电流之间存在很强的线性相关性 (ACS Catal. 2022, 12, 11, 6578–6588)。从头检查早期关键中间体 CO*、COH* 和 CHO* 的热力学,我们发现 CO* → CHO* 是热力学控制步骤。除了仅显示 CO2R 活性线性趋势的一般 CO 吸附能之外,我们还表明 CO* → CHO* 的反应自由能是 Cu 刻面整体 CO2R 活度的描述符,因为它显示了与实验电流的火山关系。重要的是,我们表明 Cu 终端的高阶跃密度和扭结密度不仅增强了 CO 吸附强度,而且还调节了 CO* → CHO* 途径,分别在 (941) 和 (741) 分面中所示。此外,我们解释说,(741) 的高活性是由于与其他 Cu 表面相比,其析氢反应活性相对较低。
更新日期:2024-11-10
中文翻译:

Cu 刻面上二氧化碳还原的电化学电流的计算描述符
计算筛选对于设计用于二氧化碳 (CO2R) 还原的高效电化学催化剂至关重要,这些催化剂可产生有价值的碳氢化合物和含氧化合物。在此,利用 17 个 Cu 终端上 CO 吸附能 ΔECO 的密度泛函理论计算,我们发现 ΔECO 与实验测量的 CO2R 电化学电流之间存在很强的线性相关性 (ACS Catal. 2022, 12, 11, 6578–6588)。从头检查早期关键中间体 CO*、COH* 和 CHO* 的热力学,我们发现 CO* → CHO* 是热力学控制步骤。除了仅显示 CO2R 活性线性趋势的一般 CO 吸附能之外,我们还表明 CO* → CHO* 的反应自由能是 Cu 刻面整体 CO2R 活度的描述符,因为它显示了与实验电流的火山关系。重要的是,我们表明 Cu 终端的高阶跃密度和扭结密度不仅增强了 CO 吸附强度,而且还调节了 CO* → CHO* 途径,分别在 (941) 和 (741) 分面中所示。此外,我们解释说,(741) 的高活性是由于与其他 Cu 表面相比,其析氢反应活性相对较低。

































 京公网安备 11010802027423号
京公网安备 11010802027423号