当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design, synthesis, and X-ray structural studies of a series of highly potent, selective, and drug-like G protein-coupled receptor kinase 5 inhibitors
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-08 , DOI: 10.1016/j.ejmech.2024.117024 Arun K. Ghosh, Yueyi Chen, Ranjith Kumar Gadi, Amol Sonawane, Sandali Piladuwa Gamage, JohnJ.G. Tesmer
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-08 , DOI: 10.1016/j.ejmech.2024.117024 Arun K. Ghosh, Yueyi Chen, Ranjith Kumar Gadi, Amol Sonawane, Sandali Piladuwa Gamage, JohnJ.G. Tesmer
|
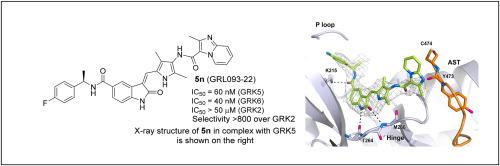
G protein-coupled receptor kinase 5 (GRK5) has emerged as a potential drug development target against heart failure and cancer. A close homolog, GRK6 represents a therapeutic target for multiple myeloma. We have rationally designed a series of highly selective, potent, noncovalent, and drug-like GRK5 inhibitors. Several inhibitors exhibited low nanomolar GRK5 inhibition and high selectivity over GRK2, and, surprisingly, some were selective for GRK6. We determined high-resolution X-ray crystal structures of several inhibitors in complex with GRK5, which provide molecular insights into the ligand-binding site interactions responsible for GRK5 selectivity and potency.
中文翻译:

一系列高效、选择性和药物样 G 蛋白偶联受体激酶 5 抑制剂的设计、合成和 X 射线结构研究
G 蛋白偶联受体激酶 5 (GRK5) 已成为治疗心力衰竭和癌症的潜在药物开发靶点。GRK6 是一种接近的同源物,代表多发性骨髓瘤的治疗靶点。我们合理设计了一系列高选择性、强效、非共价和类药的 GRK5 抑制剂。几种抑制剂表现出低纳摩尔 GRK5 抑制和对 GRK2 的高选择性,令人惊讶的是,有些抑制剂对 GRK6 具有选择性。我们确定了与 GRK5 复合物中几种抑制剂的高分辨率 X 射线晶体结构,这为负责 GRK5 选择性和效力的配体结合位点相互作用提供了分子见解。
更新日期:2024-11-08
中文翻译:

一系列高效、选择性和药物样 G 蛋白偶联受体激酶 5 抑制剂的设计、合成和 X 射线结构研究
G 蛋白偶联受体激酶 5 (GRK5) 已成为治疗心力衰竭和癌症的潜在药物开发靶点。GRK6 是一种接近的同源物,代表多发性骨髓瘤的治疗靶点。我们合理设计了一系列高选择性、强效、非共价和类药的 GRK5 抑制剂。几种抑制剂表现出低纳摩尔 GRK5 抑制和对 GRK2 的高选择性,令人惊讶的是,有些抑制剂对 GRK6 具有选择性。我们确定了与 GRK5 复合物中几种抑制剂的高分辨率 X 射线晶体结构,这为负责 GRK5 选择性和效力的配体结合位点相互作用提供了分子见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号