当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Novel frontiers through nitrogen substitution at 6th, 10th and 11th position of artemisinin: Synthetic approaches and antimalarial activity
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-07 , DOI: 10.1016/j.ejmech.2024.117032 Priyanka Yadav, Varun Rawat, Shalini Kaushik Love, Ved Prakash Verma
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-07 , DOI: 10.1016/j.ejmech.2024.117032 Priyanka Yadav, Varun Rawat, Shalini Kaushik Love, Ved Prakash Verma
|
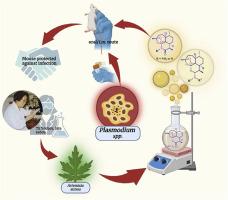
Malaria pertains to an array of catastrophic illnesses spurred on by the Plasmodium spp. Artemisinin (ART) is currently prescribed in conjunction with another medication as part of therapeutic regimens for acute malaria. These currently prescribed pharmaceuticals have been around for a while, even after lack of required thermos-metabolic stabilities, alongside fresh proclaims about surfacing resistance and neurotoxicity linked with sequential administration of such combination therapies. Over the years, ARTs seem to have gained popularity through the accelerated reduction in parasitaemia, thus dictating use of differentially stable ART derivatives, in combination or alone, to control the proliferation of malaria. The endoperoxide bridge in the ART pharmacophore plays a non-negotiable role in its action against multiple stages in the parasitic life cycle. However, shorter half-lives and limited bioavailability tend to open doors for another class of endoperoxides. Nitrogen substitution at 6th, 10th and 11th positions of ART draws attention as the best replacements through their disparate stabilities and inability to demonstrate in vivo hydrolytic decomposition into DHA. Discussions pertaining such azaartemisinins and aminoartemisinins reported over the past 30 years have been strongly focused upon, on account of their synthetic methodologies and antimalarial efficacies, in order to assign future candidature to the meritorious moiety.
中文翻译:

青蒿素第 6、10 和 11 位氮取代的新前沿:合成方法和抗疟活性
疟疾与疟原虫属引发的一系列灾难性疾病有关。青蒿素 (ART) 目前与另一种药物一起开具,作为急性疟疾治疗方案的一部分。这些目前的处方药已经存在了一段时间,即使在缺乏所需的热代谢稳定性之后,以及关于与连续给药此类联合疗法相关的浮出面耐药和神经毒性的新声明。多年来,ART 似乎通过加速减少寄生虫血症而广受欢迎,因此需要联合或单独使用差异稳定的 ART 衍生物来控制疟疾的扩散。ART 药效团中的内过氧化物桥在其对寄生虫生命周期中多个阶段的作用中起着不可协商的作用。然而,较短的半衰期和有限的生物利用度往往为另一类内过氧化物打开了大门。ART 第 6、10 和 11 位的氮取代因其不同的稳定性和无法证明体内水解分解成 DHA 而作为最佳替代品而受到关注。过去 30 年报道的有关此类氮杂杆菌素和氨基青蒿素的讨论一直受到强烈关注,因为它们的合成方法和抗疟功效,以便为功勋部分分配未来的候选资格。
更新日期:2024-11-07
中文翻译:

青蒿素第 6、10 和 11 位氮取代的新前沿:合成方法和抗疟活性
疟疾与疟原虫属引发的一系列灾难性疾病有关。青蒿素 (ART) 目前与另一种药物一起开具,作为急性疟疾治疗方案的一部分。这些目前的处方药已经存在了一段时间,即使在缺乏所需的热代谢稳定性之后,以及关于与连续给药此类联合疗法相关的浮出面耐药和神经毒性的新声明。多年来,ART 似乎通过加速减少寄生虫血症而广受欢迎,因此需要联合或单独使用差异稳定的 ART 衍生物来控制疟疾的扩散。ART 药效团中的内过氧化物桥在其对寄生虫生命周期中多个阶段的作用中起着不可协商的作用。然而,较短的半衰期和有限的生物利用度往往为另一类内过氧化物打开了大门。ART 第 6、10 和 11 位的氮取代因其不同的稳定性和无法证明体内水解分解成 DHA 而作为最佳替代品而受到关注。过去 30 年报道的有关此类氮杂杆菌素和氨基青蒿素的讨论一直受到强烈关注,因为它们的合成方法和抗疟功效,以便为功勋部分分配未来的候选资格。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号