当前位置:
X-MOL 学术
›
J. Chem. Theory Comput.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ion-Specific Surface Tension of Aqueous Electrolyte Solutions: Analytical Insights from a Restricted Primitive Model
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.jctc.4c01168 Tiejun Xiao, Yun Zhou, Huijun Jiang
Journal of Chemical Theory and Computation ( IF 5.7 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.jctc.4c01168 Tiejun Xiao, Yun Zhou, Huijun Jiang
|
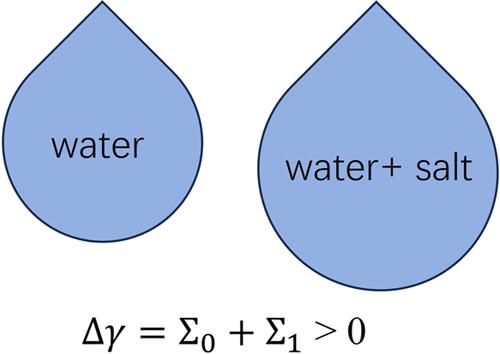
The ion-specific surface tension of aqueous electrolyte solutions is of fundamental importance in physical chemistry, solution chemistry, electrochemistry, and biochemistry, yet it remains a challenge to be qualitatively predicted. In this work, an analytical theory of ion-specific surface tension is developed. By modeling the solution to a restricted primitive model (RPM), the surface tension increment of the electrolyte solution reduces to the surface tension of the RPM, which is further determined analytically by using the integral equation theory and the morphological thermodynamics theory. According to our formula, the surface tension increment of the electrolyte solution consists of a dominant and positive hard sphere contribution, which depends on the salt concentration, and a secondary electrostatic contribution, which depends on the inverse Debye length and the salt concentration. Our theory is applied to 1:1, 2:2, 1:2, 2:1, 3:1, and 3:2 electrolyte solutions with typical salt concentrations up to several mol/L and compared with experimental data. Without introducing any adjustable parameters, our theory leads to a good prediction of the surface tension increment of more than 50 kinds of aqueous solutions. Such a good agreement demonstrates the great potential of our theory for a fundamental understanding of specific ion effects in a variety of electrolyte solutions.
中文翻译:

电解质水溶液的离子特异性表面张力:来自受限基元模型的分析见解
电解质水溶液的离子特异性表面张力在物理化学、溶液化学、电化学和生物化学中至关重要,但定性预测仍然是一个挑战。在这项工作中,开发了离子特异性表面张力的解析理论。通过将解建模为受限原始模型 (RPM),电解质溶液的表面张力增量减小到 RPM 的表面张力,该表面张力通过使用积分方程理论和形态热力学理论进一步解析确定。根据我们的公式,电解质溶液的表面张力增量由一个占主导地位的正硬球贡献(取决于盐浓度)和一个次级静电贡献(取决于反德拜长度和盐浓度)组成。我们的理论应用于 1:1、2:2、1:2、2:1、3:1 和 3:2 电解质溶液,典型盐浓度高达几 mol/L,并与实验数据进行比较。在不引入任何可调参数的情况下,我们的理论可以很好地预测 50 多种水溶液的表面张力增量。这种很好的一致性证明了我们的理论在从根本上理解各种电解质溶液中的特定离子效应方面的巨大潜力。
更新日期:2024-11-08
中文翻译:

电解质水溶液的离子特异性表面张力:来自受限基元模型的分析见解
电解质水溶液的离子特异性表面张力在物理化学、溶液化学、电化学和生物化学中至关重要,但定性预测仍然是一个挑战。在这项工作中,开发了离子特异性表面张力的解析理论。通过将解建模为受限原始模型 (RPM),电解质溶液的表面张力增量减小到 RPM 的表面张力,该表面张力通过使用积分方程理论和形态热力学理论进一步解析确定。根据我们的公式,电解质溶液的表面张力增量由一个占主导地位的正硬球贡献(取决于盐浓度)和一个次级静电贡献(取决于反德拜长度和盐浓度)组成。我们的理论应用于 1:1、2:2、1:2、2:1、3:1 和 3:2 电解质溶液,典型盐浓度高达几 mol/L,并与实验数据进行比较。在不引入任何可调参数的情况下,我们的理论可以很好地预测 50 多种水溶液的表面张力增量。这种很好的一致性证明了我们的理论在从根本上理解各种电解质溶液中的特定离子效应方面的巨大潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号