当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Veliparib: Analytical Tools and Process Design Strategies for the Control of Mutagenic Impurities and Other Drug Substance Critical Quality Attributes
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-08 , DOI: 10.1021/acs.oprd.4c00328 Rajarathnam E. Reddy, David M. Barnes, Adam P. Schellinger, Travis B. Dunn, Lawrence Kolaczkowski, Wayne A. Pritts, Yao-En David Li, Samrat Mukherjee, Andrew Staley
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-08 , DOI: 10.1021/acs.oprd.4c00328 Rajarathnam E. Reddy, David M. Barnes, Adam P. Schellinger, Travis B. Dunn, Lawrence Kolaczkowski, Wayne A. Pritts, Yao-En David Li, Samrat Mukherjee, Andrew Staley
|
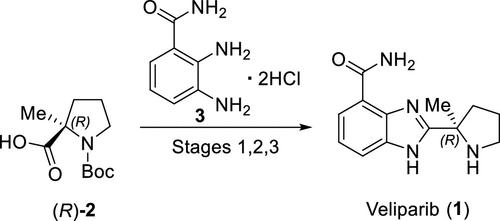
(R)-Veliparib (ABT-888) is a poly(ADP-ribose)polymerase (PARP) inhibitor that is being investigated for the treatment of a broad spectrum of oncology indications, including BRCA1/2-mutated breast cancer and other solid tumors. The (R)-veliparib process consists of three stages utilizing two proposed regulatory starting materials, (R)-Boc-2-methylproline and 2,3-diaminobenzamide dihydrochloride, with two isolated intermediates. The drug substance control strategy, which was established based on a combination of analytical tools and uniquely designed manufacturing process and unit operations, provides robust controls for mutagenic and other impurities and ensures that (R)-veliparib drug substance consistently meets all critical quality attributes (CQAs) and acceptance criteria. The purpose of this article is to provide details of how the (R)-veliparib control strategy for the selected CQAs was cross-functionally developed using analytical measurement tools and specially designed unit operations.
中文翻译:

Veliparib:用于控制致突变杂质和其他原料药关键质量属性的分析工具和工艺设计策略
(R)-Veliparib (ABT-888) 是一种聚 (ADP-核糖) 聚合酶 (PARP) 抑制剂,正在研究用于治疗多种肿瘤适应症,包括 BRCA1/2 突变的乳腺癌和其他实体瘤。(R)-veliparib 工艺由三个阶段组成,利用两种拟议的调节起始材料,(R)-Boc-2-甲基脯氨酸和 2,3-二氨基苯甲酰胺二盐酸盐,以及两种分离的中间体。原料药控制策略基于分析工具和独特设计的生产工艺和单元操作的组合而建立,为诱变性杂质和其他杂质提供稳健的控制,并确保 (R)-veliparib 原料药始终符合所有关键质量属性 (CQA) 和验收标准。本文的目的是详细介绍如何使用分析测量工具和专门设计的单元操作跨功能开发所选 CQA 的 (R)-veliparib 控制策略。
更新日期:2024-11-08
中文翻译:

Veliparib:用于控制致突变杂质和其他原料药关键质量属性的分析工具和工艺设计策略
(R)-Veliparib (ABT-888) 是一种聚 (ADP-核糖) 聚合酶 (PARP) 抑制剂,正在研究用于治疗多种肿瘤适应症,包括 BRCA1/2 突变的乳腺癌和其他实体瘤。(R)-veliparib 工艺由三个阶段组成,利用两种拟议的调节起始材料,(R)-Boc-2-甲基脯氨酸和 2,3-二氨基苯甲酰胺二盐酸盐,以及两种分离的中间体。原料药控制策略基于分析工具和独特设计的生产工艺和单元操作的组合而建立,为诱变性杂质和其他杂质提供稳健的控制,并确保 (R)-veliparib 原料药始终符合所有关键质量属性 (CQA) 和验收标准。本文的目的是详细介绍如何使用分析测量工具和专门设计的单元操作跨功能开发所选 CQA 的 (R)-veliparib 控制策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号