当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Synthesis of the cis-1,2-Diaryltetralin Vepdegestrant Core via an Enantioselective Intramolecular Corey-Chaykovsky Epoxidation
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-08 , DOI: 10.1021/acs.oprd.4c00379 David J. Bernhardson, Sarai Lara-Boykin, James Christopher McWilliams, Yexenia Nieves-Quinones, Liam S. Sharninghausen, Robert A. Singer, Zheng Wang, Zebediah C. Girvin
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-08 , DOI: 10.1021/acs.oprd.4c00379 David J. Bernhardson, Sarai Lara-Boykin, James Christopher McWilliams, Yexenia Nieves-Quinones, Liam S. Sharninghausen, Robert A. Singer, Zheng Wang, Zebediah C. Girvin
|
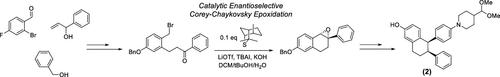
An asymmetric synthesis of cis-1,2-diaryltetralin (2), a key chiral intermediate in the synthesis of Vepdegestrant (1), an orally available PROTAC being evaluated for the treatment of ER+/HER2- breast cancer, is reported. Chirality is initially introduced via a catalytic enantioselective intramolecular Corey-Chaykovsky epoxidation utilizing isothiocineole, a chiral sulfide. Although several asymmetric intermolecular epoxidations using chiral sulfides have been reported, our approach represents a unique intramolecular variant. The subsequent introduction of the cis-1,2-diaryl motif is achieved via diastereoselective hydrogenation.
中文翻译:

通过对映选择性分子内 Corey-Chaykovsky 环氧化合成顺式-1,2-二芳基四烷基四蛋白 Vepdegestrant 核心
据报道,顺式-1,2-二芳基四蛋白 (2) 的不对称合成是合成 Vepdegestrant (1) 的关键手性中间体,Vepdegestrant 是一种口服 PROTAC,正在评估用于治疗 ER+/HER2- 乳腺癌。手性最初是通过利用异硫酰酰(一种手性硫化物)的催化对映选择性分子内 Corey-Chaykovsky 环氧化引入的。尽管已经报道了几种使用手性硫化物的不对称分子间环氧化,但我们的方法代表了一种独特的分子内变体。顺式-1,2-二芳基基序的后续引入是通过非对映选择性氢化实现的。
更新日期:2024-11-08
中文翻译:

通过对映选择性分子内 Corey-Chaykovsky 环氧化合成顺式-1,2-二芳基四烷基四蛋白 Vepdegestrant 核心
据报道,顺式-1,2-二芳基四蛋白 (2) 的不对称合成是合成 Vepdegestrant (1) 的关键手性中间体,Vepdegestrant 是一种口服 PROTAC,正在评估用于治疗 ER+/HER2- 乳腺癌。手性最初是通过利用异硫酰酰(一种手性硫化物)的催化对映选择性分子内 Corey-Chaykovsky 环氧化引入的。尽管已经报道了几种使用手性硫化物的不对称分子间环氧化,但我们的方法代表了一种独特的分子内变体。顺式-1,2-二芳基基序的后续引入是通过非对映选择性氢化实现的。






























 京公网安备 11010802027423号
京公网安备 11010802027423号