当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermodynamically Controlled and Industrially Viable Telescopic Process for the Synthesis of Fluazuron
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.oprd.4c00345 Dattatray Patil, Rakesh R. Ganorkar, Ramakant Kardile, Madhavrao Bhoite, Amol Jadhav, Rutuja Gundal, Garbapu Suresh
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.oprd.4c00345 Dattatray Patil, Rakesh R. Ganorkar, Ramakant Kardile, Madhavrao Bhoite, Amol Jadhav, Rutuja Gundal, Garbapu Suresh
|
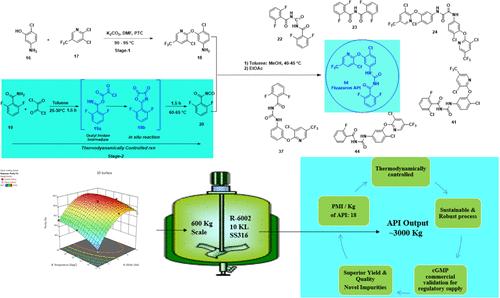
Fluazuron N-[(4-chloro-3-[3-chloro-5-(trifluoromethyl)pyridine-2-yl]oxy phenyl]carbamoyl]-2,6-difluorobenzamide (14) is a noteworthy antiparasitic veterinary medicine belonging to the class of benzoyl phenyl urea derivatives, a class of chitin synthesis inhibitors. The commercial-scale synthesis, which is compliant with current regulatory requirements, particularly purity and impurity profiles, is not well established. Therefore, a robust and sustainable manufacturing process is essential to manufacture and supply fluazuron or any drug substance, for that matter, meeting all criteria. In this work, a safe, scalable, economic, and sustainable process was described through a robust in situ protocol for the bottleneck isocyanate intermediate (20) to manufacture a substantially pure fluazuron active pharmaceutical ingredient (API) with >99.5% HPLC purity and a yield of >78% overall. This large-scale GMP manufacturing process was established by implementing DoE tools and principles of green chemistry like process mass intensity assessment (PMI) and the “3Rs” principle (reduce/reuse/recycle) to attain the “3Ps” sustainability target (profit/people/planet). The developed process technology was successfully validated under cGMP plant conditions on a scale of 600 kg batch size to supply the fluazuron API (14) across the globe for veterinary use. This process is commercially friendly and environmentally benign. Furthermore, several process-related impurities were identified, synthesized, characterized, and studied for their purging capability. According to the SciFinder database, there are two new impurities (23 and 24), which are structurally similar to the fluazuron API, that could lead to the discovery of new biological applications in both animal and human drug development.
中文翻译:

用于合成氟唑隆的热力学控制和工业上可行的伸缩工艺
氟唑隆 N-[(4-氯-3-[3-氯-5-(三氟甲基)吡啶-2-基]氧基苯基]氨基甲酰基]-2,6-二氟苯甲酰胺 (14) 是一种值得注意的抗寄生虫兽药,属于苯甲酰苯基脲衍生物类,一类甲壳素合成抑制剂。符合当前监管要求(尤其是纯度和杂质概况)的商业规模合成尚未完全确立。因此,稳健且可持续的制造工艺对于制造和供应氟唑隆或任何原料药至关重要,就此而言,要满足所有标准。在这项工作中,通过瓶颈异氰酸酯中间体 (20) 的稳健原位方案描述了一种安全、可扩展、经济和可持续的工艺,以制造具有 >99.5% HPLC 纯度和 >78% 总体产率的基本上纯度的氟唑隆活性药物成分 (API)。这种大规模的 GMP 生产工艺是通过实施 DoE 工具和绿色化学原则(如过程质量强度评估 (PMI) 和“3R”原则(减少/再利用/回收))建立的,以实现“3Ps”可持续发展目标(利润/人类/地球)。开发的工艺技术在 cGMP 工厂条件下以 600 kg 批量大小的规模成功验证,可在全球范围内供应氟唑隆 API (14) 用于兽医。这个过程在商业上是友好的,对环境无害。此外,还鉴定、合成、表征并研究了几种与工艺相关的杂质的吹扫能力。 根据 SciFinder 数据库,有两种新的杂质(23 和 24),它们在结构上与氟唑隆 API 相似,这可能导致在动物和人类药物开发中发现新的生物学应用。
更新日期:2024-11-09
中文翻译:

用于合成氟唑隆的热力学控制和工业上可行的伸缩工艺
氟唑隆 N-[(4-氯-3-[3-氯-5-(三氟甲基)吡啶-2-基]氧基苯基]氨基甲酰基]-2,6-二氟苯甲酰胺 (14) 是一种值得注意的抗寄生虫兽药,属于苯甲酰苯基脲衍生物类,一类甲壳素合成抑制剂。符合当前监管要求(尤其是纯度和杂质概况)的商业规模合成尚未完全确立。因此,稳健且可持续的制造工艺对于制造和供应氟唑隆或任何原料药至关重要,就此而言,要满足所有标准。在这项工作中,通过瓶颈异氰酸酯中间体 (20) 的稳健原位方案描述了一种安全、可扩展、经济和可持续的工艺,以制造具有 >99.5% HPLC 纯度和 >78% 总体产率的基本上纯度的氟唑隆活性药物成分 (API)。这种大规模的 GMP 生产工艺是通过实施 DoE 工具和绿色化学原则(如过程质量强度评估 (PMI) 和“3R”原则(减少/再利用/回收))建立的,以实现“3Ps”可持续发展目标(利润/人类/地球)。开发的工艺技术在 cGMP 工厂条件下以 600 kg 批量大小的规模成功验证,可在全球范围内供应氟唑隆 API (14) 用于兽医。这个过程在商业上是友好的,对环境无害。此外,还鉴定、合成、表征并研究了几种与工艺相关的杂质的吹扫能力。 根据 SciFinder 数据库,有两种新的杂质(23 和 24),它们在结构上与氟唑隆 API 相似,这可能导致在动物和人类药物开发中发现新的生物学应用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号