当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Adsorbability of a Wide Range of Per- and Polyfluoroalkyl Substances on Granular Activated Carbon, Ion Exchange Resin, and Surface Modified Clay
Water Research ( IF 11.4 ) Pub Date : 2024-11-09 , DOI: 10.1016/j.watres.2024.122774 Bahareh Tajdini, Hooman Vatankhah, Ethan R. Pezoulas, Chuhui Zhang, Christopher P Higgins, Christopher Bellona
Water Research ( IF 11.4 ) Pub Date : 2024-11-09 , DOI: 10.1016/j.watres.2024.122774 Bahareh Tajdini, Hooman Vatankhah, Ethan R. Pezoulas, Chuhui Zhang, Christopher P Higgins, Christopher Bellona
|
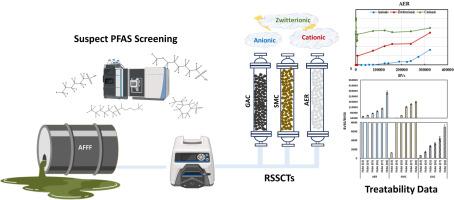
The increased detection of understudied per- and polyfluoroalkyl substances (PFAS) in environmental matrices has highlighted the need to evaluate the treatability of a wide-range of PFAS by sorption-based processes. This study investigated the efficacy of three commercial adsorbents (i.e., granular activated carbon (GAC), surface modified clay (SMC), and anionic exchange resin (AER)) for the removal of a wide range of cationic, zwitterionic, and anionic PFAS from an aqueous film forming foam (AFFF)-impacted groundwater employing rapid small-scale column tests (RSSCTs) coupled with high resolution mass spectrometry (HRMS) and suspect screening analysis (SQ). AER exhibited later breakthrough times for the majority of anionic and zwitterionic PFAS compared to SMC and GAC. However, both AER and SMC exhibited negligible removal of cationic PFAS presumably due to the reliance of these adsorbents on electrostatic interactions and the counteraction of hydrophobic forces caused by the repulsion between cationic PFAS and positively charged surfaces of AER and SMC. GAC, being a non-selective adsorbent, was largely unaffected by the ionic charge of the evaluated PFAS with molecular structure having a bigger impact on adsorbability. The detection of a variety of PFAS classes in the investigated AFFF-impacted groundwater enabled assessment of the relative impact of chemical structure on adsorptive removal of PFAS. Chain-length dependent adsorption was observed across all investigated anionic and zwitterionic PFAS classes. The PFAS structures possessing hydroxyl and/or methyl functional groups exhibited later breakthrough times compared to their homologues lacking these functional groups and cyclic/unsaturated structures were removed less efficiently compared to their linear/saturated homologues. In the case of perfluoroalkyl acid (PFAA)-derivative structures, hydrogen-substituted classes (i.e., H-PFAAs) were removed more efficiency than PFAAs while keto-substituted structures (i.e., K-PFSA) and pentahydrido-fluoroalkane sulfates (PeH-FAOS) exhibited lower adsorbability compared to PFAAs for all adsorbents. Oxa-PFAAs (O-PFSA; isomer class of PFA-OS) on the other hand demonstrated higher adsorbability compared to PFAAs in the case of AER-like adsorbents, while this trend was reversed for GAC.
中文翻译:

多种全氟和多氟烷基物质在颗粒活性炭、离子交换树脂和表面改性粘土上的吸附性
环境基质中研究不足的全氟烷基和多氟烷基物质 (PFAS) 的检出率增加,这凸显了通过基于吸附的工艺评估各种 PFAS 的可处理性的必要性。本研究调查了三种商业吸附剂(即颗粒活性炭 (GAC)、表面改性粘土 (SMC) 和阴离子交换树脂 (AER))使用快速小规模柱测试 (RSSCT) 结合高分辨率质谱 (HRMS) 和可疑筛查分析 (SQ) 从受成膜泡沫 (AFFF) 影响的地下水中去除各种阳离子、两性离子和阴离子 PFAS 的功效。与 SMC 和 GAC 相比,大多数阴离子和两性离子 PFAS 的突破时间较晚。然而,AER 和 SMC 对阳离子 PFAS 的去除作用可以忽略不计,这可能是由于这些吸附剂依赖于静电相互作用,以及阳离子 PFAS 与 AER 和 SMC 带正电荷的表面之间的排斥作用所引起的疏水力的抵消作用。GAC 是一种非选择性吸附剂,在很大程度上不受所评估 PFAS 的离子电荷的影响,分子结构对吸附性的影响更大。在所调查的受 AFFF 影响的地下水中检测到各种 PFAS 类别,从而能够评估化学结构对 PFAS 吸附去除的相对影响。在所有研究的阴离子和两性离子 PFAS 类别中观察到链长依赖性吸附。 与缺乏羟基和/或甲基官能团的同系物相比,具有羟基和/或甲基官能团的 PFAS 结构表现出较晚的突破时间,并且与线性/饱和同系物相比,环状/不饱和结构的去除效率较低。在全氟烷基酸 (PFAA) 衍生物结构的情况下,氢取代类(即 H-PFAA)的去除效率高于 PFAA,而酮取代结构(即 K-PFSA)和五氢基氟烷烃硫酸盐 (PeH-FAOS) 对所有吸附剂的吸附性均低于 PFAA。另一方面,在类 AER 吸附剂的情况下,与 PFAA 相比,Oxa-PFAA(O-PFSA;PFA-OS 的异构体类别)表现出更高的吸附性,而 GAC 的这一趋势则相反。
更新日期:2024-11-10
中文翻译:

多种全氟和多氟烷基物质在颗粒活性炭、离子交换树脂和表面改性粘土上的吸附性
环境基质中研究不足的全氟烷基和多氟烷基物质 (PFAS) 的检出率增加,这凸显了通过基于吸附的工艺评估各种 PFAS 的可处理性的必要性。本研究调查了三种商业吸附剂(即颗粒活性炭 (GAC)、表面改性粘土 (SMC) 和阴离子交换树脂 (AER))使用快速小规模柱测试 (RSSCT) 结合高分辨率质谱 (HRMS) 和可疑筛查分析 (SQ) 从受成膜泡沫 (AFFF) 影响的地下水中去除各种阳离子、两性离子和阴离子 PFAS 的功效。与 SMC 和 GAC 相比,大多数阴离子和两性离子 PFAS 的突破时间较晚。然而,AER 和 SMC 对阳离子 PFAS 的去除作用可以忽略不计,这可能是由于这些吸附剂依赖于静电相互作用,以及阳离子 PFAS 与 AER 和 SMC 带正电荷的表面之间的排斥作用所引起的疏水力的抵消作用。GAC 是一种非选择性吸附剂,在很大程度上不受所评估 PFAS 的离子电荷的影响,分子结构对吸附性的影响更大。在所调查的受 AFFF 影响的地下水中检测到各种 PFAS 类别,从而能够评估化学结构对 PFAS 吸附去除的相对影响。在所有研究的阴离子和两性离子 PFAS 类别中观察到链长依赖性吸附。 与缺乏羟基和/或甲基官能团的同系物相比,具有羟基和/或甲基官能团的 PFAS 结构表现出较晚的突破时间,并且与线性/饱和同系物相比,环状/不饱和结构的去除效率较低。在全氟烷基酸 (PFAA) 衍生物结构的情况下,氢取代类(即 H-PFAA)的去除效率高于 PFAA,而酮取代结构(即 K-PFSA)和五氢基氟烷烃硫酸盐 (PeH-FAOS) 对所有吸附剂的吸附性均低于 PFAA。另一方面,在类 AER 吸附剂的情况下,与 PFAA 相比,Oxa-PFAA(O-PFSA;PFA-OS 的异构体类别)表现出更高的吸附性,而 GAC 的这一趋势则相反。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号