当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating the Pivotal Role of Acid-Catalyzed Hydration in Electrochemical Carbon Corrosion
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-08 , DOI: 10.1021/acscatal.4c05547 Seunghoon Lee, Haesol Kim, Minho M. Kim, Tae Kyung Ko, Hyung Min Chi, Hyungjun Kim, Chang Hyuck Choi
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-08 , DOI: 10.1021/acscatal.4c05547 Seunghoon Lee, Haesol Kim, Minho M. Kim, Tae Kyung Ko, Hyung Min Chi, Hyungjun Kim, Chang Hyuck Choi
|
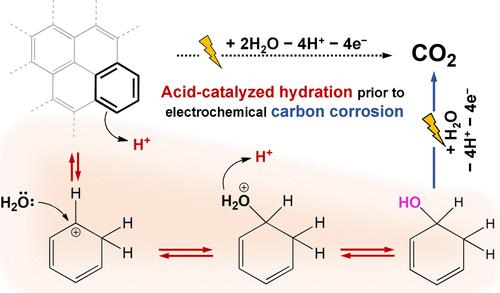
Carbon, with its high electrical conductivity and large surface area, enables the efficient dispersion and utilization of catalytic entities, contributing to the cost-effective development of electrochemical systems for a future energy economy. However, the longevity of these systems is often compromised by carbon corrosion, the fundamental details of which unfortunately remain largely unknown. Here, we elucidate that carbon corrosion is initiated by a covalent addition reaction that chemically breaks the sp2 carbon network, prior to electrochemical oxidation steps. Online differential electrochemical mass spectroscopy and post-mortem X-ray photoelectron spectroscopy unveil the pseudozeroth- and first-order reaction kinetics in the proton concentration and oxygen coverage on the carbon surface, respectively, allowing us to suggest acid-catalyzed hydration with carbocation formation as the initial step in carbon corrosion. The proposed mechanism is further evidenced by the decreased carbon corrosion rate in the presence of the carbocation scavenger, methanol, and by the evolution of the C18O16O product during the corrosion of carbon, pretreated in acid solution prepared with the 18O-isotope of water. Based on these findings, previous empirical understandings, pH-dependent and site-specific (defect, edge, etc.) carbon corrosion characteristics, can be successfully explained, bringing potential avenues for developing rational strategies to mitigate carbon corrosion.
中文翻译:

阐明酸催化水合在电化学碳腐蚀中的关键作用
碳具有高导电性和大表面积,能够有效地分散和利用催化实体,有助于经济高效地开发未来能源经济的电化学系统。然而,这些系统的使用寿命经常受到碳腐蚀的影响,不幸的是,其基本细节在很大程度上仍然未知。在这里,我们阐明了碳腐蚀是由共价加成反应引发的,该反应在电化学氧化步骤之前以化学方式破坏 sp2 碳网络。在线差分电化学质谱和死后 X 射线光电子能谱分别揭示了碳表面质子浓度和氧覆盖率的伪零级和一级反应动力学,使我们能够提出酸催化水合和碳阳离子形成作为碳腐蚀的第一步。在碳阳离子清除剂甲醇存在下碳腐蚀速率降低,以及在用水的 18O-同位素制备的酸溶液预处理的碳腐蚀过程中 C18O16O 产物的释放进一步证明了所提出的机制。基于这些发现,可以成功地解释以前的实证理解,即 pH 依赖性和位置特异性(缺陷、边缘等)碳腐蚀特性,为制定合理的策略来减轻碳腐蚀带来潜在的途径。
更新日期:2024-11-09
中文翻译:

阐明酸催化水合在电化学碳腐蚀中的关键作用
碳具有高导电性和大表面积,能够有效地分散和利用催化实体,有助于经济高效地开发未来能源经济的电化学系统。然而,这些系统的使用寿命经常受到碳腐蚀的影响,不幸的是,其基本细节在很大程度上仍然未知。在这里,我们阐明了碳腐蚀是由共价加成反应引发的,该反应在电化学氧化步骤之前以化学方式破坏 sp2 碳网络。在线差分电化学质谱和死后 X 射线光电子能谱分别揭示了碳表面质子浓度和氧覆盖率的伪零级和一级反应动力学,使我们能够提出酸催化水合和碳阳离子形成作为碳腐蚀的第一步。在碳阳离子清除剂甲醇存在下碳腐蚀速率降低,以及在用水的 18O-同位素制备的酸溶液预处理的碳腐蚀过程中 C18O16O 产物的释放进一步证明了所提出的机制。基于这些发现,可以成功地解释以前的实证理解,即 pH 依赖性和位置特异性(缺陷、边缘等)碳腐蚀特性,为制定合理的策略来减轻碳腐蚀带来潜在的途径。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号