当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Csp3–Csp2 Coupling of Isonitriles and (Hetero)arenes through a Photoredox-Catalyzed Double Decyanation Process
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-08 , DOI: 10.1021/acscatal.4c06269 María Martín, Rafael Martín Romero, Chiara Portolani, Mariola Tortosa
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-08 , DOI: 10.1021/acscatal.4c06269 María Martín, Rafael Martín Romero, Chiara Portolani, Mariola Tortosa
|
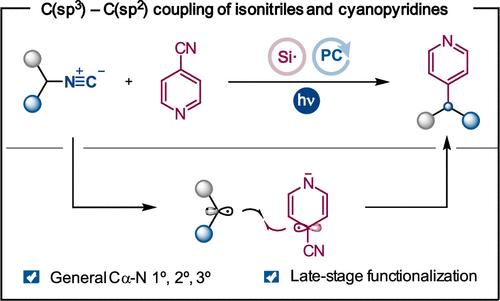
Herein, we demonstrate the ability of isonitriles to be used as alkyl radical precursors in a photoredox-catalyzed transformation involving selective C–N cleavage and Csp3–Csp2 bond formation. This protocol allows for the preparation of functionalized heteroarenes from readily available isonitriles through a decyanation process. The reaction is general for primary, secondary, and tertiary substrates, including amino acid derivatives and druglike molecules.
中文翻译:

异腈和(杂)芳烃通过光氧化还原催化的双脱氰过程的 Csp3–Csp2 偶联
在此,我们证明了异腈在光氧化还原催化转化中用作烷基自由基前体的能力,涉及选择性 C-N 切割和 Csp3-Csp 2 键形成。该方案允许通过脱氰工艺从现成的异腈制备功能化的杂芳烃。该反应通常适用于一级、二级和三级底物,包括氨基酸衍生物和药物样分子。
更新日期:2024-11-09
中文翻译:

异腈和(杂)芳烃通过光氧化还原催化的双脱氰过程的 Csp3–Csp2 偶联
在此,我们证明了异腈在光氧化还原催化转化中用作烷基自由基前体的能力,涉及选择性 C-N 切割和 Csp3-Csp 2 键形成。该方案允许通过脱氰工艺从现成的异腈制备功能化的杂芳烃。该反应通常适用于一级、二级和三级底物,包括氨基酸衍生物和药物样分子。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号