当前位置:
X-MOL 学术
›
Environ. Sci.: Nano
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical study of p-block metal–nitrogen–carbon single-atom catalysts for heterogeneous Fenton-like reaction
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-11-08 , DOI: 10.1039/d4en00778f Chen Zhou, Haobin Tan, Shengbo Wang, Qiang Liu, Zhenhui Xu, Peng Zhang, Chun Hu
Environmental Science: Nano ( IF 5.8 ) Pub Date : 2024-11-08 , DOI: 10.1039/d4en00778f Chen Zhou, Haobin Tan, Shengbo Wang, Qiang Liu, Zhenhui Xu, Peng Zhang, Chun Hu
|
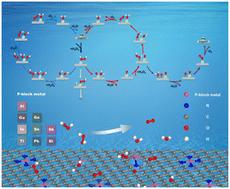
The heterogeneous Fenton-like reaction has been widely used in water purification and environmental remediation due to the highly reactive nature of hydroxyl radicals. Nevertheless, the intrinsic structure–activity relationship for heterogeneous Fenton-like reaction catalysts remains to be clarified. Metal/nitrogen/carbon (M/N/C) single-atom catalysts (SACs) provide an ideal opportunity to reveal the relationship between the structure and activity. In this work, the detailed catalytic mechanism and activity of H2O2 decomposition on p-block main-group metal/nitrogen/carbon (PM/N/C) catalysts were investigated systematically. A volcano relationship between the catalytic activity and the adsorption energies of reaction intermediates was found for H2O2 decomposition on PM/N/C SACs. PM-N2C2 and PM-C4 exhibit higher H2O2 decomposition activity than PM-N4, indicating that reducing the N/C ratio in the coordination environment can effectively adjust the catalytic activity. By altering the N/C coordination environment, it is possible to modify the p-band position of p-block main-group metal atoms in PM/N/C SACs, thereby enhancing the catalytic activity of H2O2 decomposition.
中文翻译:

p-嵌段金属-氮-碳单原子催化剂在非均相Fenton-like反应中的理论研究
由于羟基自由基的高反应性,非均相 Fenton 样反应已广泛用于水净化和环境修复。然而,非均相 Fenton 类反应催化剂的内禀构效关系仍有待阐明。金属/氮/碳 (M/N/C) 单原子催化剂 (SAC) 为揭示结构和活性之间的关系提供了理想的机会。本工作系统研究了 H2O2 在 p 嵌段主族金属/氮/碳 (PM/N/C) 催化剂上分解的详细催化机理和活性。发现 PM/N/C SAC 上 H2O2 分解反应中间体的催化活性与吸附能之间存在火山岩关系。PM-N2C2 和 PM-C4 表现出比 PM-N4 更高的 H2O2 分解活性,表明在配位环境中降低 N/C 比可以有效调节催化活性。通过改变 N/C 配位环境,可以改变 PM/N/C SAC 中 p 区主族金属原子的 p 波段位置,从而增强 H2O2 分解的催化活性。
更新日期:2024-11-08
中文翻译:

p-嵌段金属-氮-碳单原子催化剂在非均相Fenton-like反应中的理论研究
由于羟基自由基的高反应性,非均相 Fenton 样反应已广泛用于水净化和环境修复。然而,非均相 Fenton 类反应催化剂的内禀构效关系仍有待阐明。金属/氮/碳 (M/N/C) 单原子催化剂 (SAC) 为揭示结构和活性之间的关系提供了理想的机会。本工作系统研究了 H2O2 在 p 嵌段主族金属/氮/碳 (PM/N/C) 催化剂上分解的详细催化机理和活性。发现 PM/N/C SAC 上 H2O2 分解反应中间体的催化活性与吸附能之间存在火山岩关系。PM-N2C2 和 PM-C4 表现出比 PM-N4 更高的 H2O2 分解活性,表明在配位环境中降低 N/C 比可以有效调节催化活性。通过改变 N/C 配位环境,可以改变 PM/N/C SAC 中 p 区主族金属原子的 p 波段位置,从而增强 H2O2 分解的催化活性。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号