当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Investigating the formation of metal nitride complexes employing a tetradentate bis-carbene bis-phenolate ligand
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-08 , DOI: 10.1039/d4dt01765j Romain Kunert, Diego Martelino, Samyadeb Mahato, Nicholas M. Hein, Jason Pulfer, Christian Philouze, Olivier Jarjayes, Fabrice Thomas, Tim Storr
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-08 , DOI: 10.1039/d4dt01765j Romain Kunert, Diego Martelino, Samyadeb Mahato, Nicholas M. Hein, Jason Pulfer, Christian Philouze, Olivier Jarjayes, Fabrice Thomas, Tim Storr
|
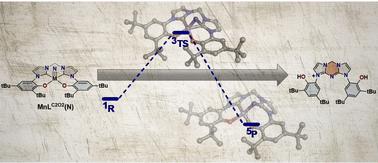
The synthesis of MnV and CrV nitride complexes of a pro-radical tetradentate bis-phenol bis-N-heterocyclic carbene ligand H2LC2O2 was investigated. Employing either azide photolysis of the MnIII precursor complex MnLC2O2(N3) or a nitride exchange reaction between MnLC2O2(Br) and the nitride exchange reagent Mnsalen(N) failed to provide a useful route to the target nitride MnLC2O2(N). Experimental results support initial formation of the target nitride MnLC2O2(N), however, the nitride rapidly inserts into a Mn–CNHC bond. A second insertion reaction results in the isolation of the doubly inserted ligand product [H2LC2O2(N)]+ in good yield. In contrast, the Cr analogue CrLC2O2(N) was readily prepared and characterized by a number of experimental methods, including X-ray crystallography. Theoretical calculations predict a lower transition state energy for nitride insertion into the M–CNHC bond for Mn in comparison to Cr, and in addition the N-inserted product is stabilized for Mn while destabilized for Cr. Natural bond order (NBO) analysis predicts that the major bonding interaction (π M![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N → σ* M–CNHC) promotes nucleophilic attack of the nitride on the carbene as the major reaction pathway. Finally, one-electron oxidation of CrLC2O2(N) affords a relatively stable cation that is characterized by experimental and theoretical analysis to be a metal-oxidized d0 CrVI species.
N → σ* M–CNHC) promotes nucleophilic attack of the nitride on the carbene as the major reaction pathway. Finally, one-electron oxidation of CrLC2O2(N) affords a relatively stable cation that is characterized by experimental and theoretical analysis to be a metal-oxidized d0 CrVI species.
中文翻译:

研究采用四齿双卡宾双酚酸酯配体的金属氮化物络合物的形成
研究了促自由基四齿双酚双-N-杂环卡宾配体 H2LC2O2 的 MnV 和 CrV 氮化物配合物的合成。采用 MnIII 前驱体复合物 MnLC2O2(N3) 的叠氮化物光解或 MnLC2O2(Br) 和氮化物交换试剂 Mnsalen(N) 之间的氮化物交换反应未能提供通往目标氮化物 MnLC2O2(N) 的有用途径。实验结果支持目标氮化物 MnLC2O2(N) 的初始形成,但是,氮化物会迅速插入 Mn-CNHC 键中。第二次插入反应以良好的产量分离双插入配体产物 [H2LC2O2(N)]+。相比之下,Cr 类似物 CrLC2O2(N) 很容易制备并通过多种实验方法表征,包括 X 射线晶体学。理论计算预测,与 Cr 相比,Nn 的氮化物插入 M-CNHC 键的过渡态能量较低,此外,N-插入产物对 Mn 稳定,而对 Cr 不稳定。自然键序 (NBO) 分析预测主要键相互作用 (π M![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N → σ* M-CNHC) 促进氮化物对卡宾的亲核攻击,作为主要反应途径。 最后,CrLC2O2(N) 的单电子氧化提供了一种相对稳定的阳离子,通过实验和理论分析,其特征是金属氧化的 d0 CrVI 物质。
N → σ* M-CNHC) 促进氮化物对卡宾的亲核攻击,作为主要反应途径。 最后,CrLC2O2(N) 的单电子氧化提供了一种相对稳定的阳离子,通过实验和理论分析,其特征是金属氧化的 d0 CrVI 物质。
更新日期:2024-11-08
![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N → σ* M–CNHC) promotes nucleophilic attack of the nitride on the carbene as the major reaction pathway. Finally, one-electron oxidation of CrLC2O2(N) affords a relatively stable cation that is characterized by experimental and theoretical analysis to be a metal-oxidized d0 CrVI species.
N → σ* M–CNHC) promotes nucleophilic attack of the nitride on the carbene as the major reaction pathway. Finally, one-electron oxidation of CrLC2O2(N) affords a relatively stable cation that is characterized by experimental and theoretical analysis to be a metal-oxidized d0 CrVI species.
中文翻译:

研究采用四齿双卡宾双酚酸酯配体的金属氮化物络合物的形成
研究了促自由基四齿双酚双-N-杂环卡宾配体 H2LC2O2 的 MnV 和 CrV 氮化物配合物的合成。采用 MnIII 前驱体复合物 MnLC2O2(N3) 的叠氮化物光解或 MnLC2O2(Br) 和氮化物交换试剂 Mnsalen(N) 之间的氮化物交换反应未能提供通往目标氮化物 MnLC2O2(N) 的有用途径。实验结果支持目标氮化物 MnLC2O2(N) 的初始形成,但是,氮化物会迅速插入 Mn-CNHC 键中。第二次插入反应以良好的产量分离双插入配体产物 [H2LC2O2(N)]+。相比之下,Cr 类似物 CrLC2O2(N) 很容易制备并通过多种实验方法表征,包括 X 射线晶体学。理论计算预测,与 Cr 相比,Nn 的氮化物插入 M-CNHC 键的过渡态能量较低,此外,N-插入产物对 Mn 稳定,而对 Cr 不稳定。自然键序 (NBO) 分析预测主要键相互作用 (π M


















































 京公网安备 11010802027423号
京公网安备 11010802027423号