当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Polyurethane foam acidolysis with carboxylic acids: acid structure dictates N-containing product distribution and kinetics
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-11-09 , DOI: 10.1039/d4py01116c Zach Westman, Manasa Perikala, Xinyi Wu, Kelsey Richardson, Christopher S. Letko, Vojtech Vlcek, Phillip Christopher, Mahdi M. Abu-Omar
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-11-09 , DOI: 10.1039/d4py01116c Zach Westman, Manasa Perikala, Xinyi Wu, Kelsey Richardson, Christopher S. Letko, Vojtech Vlcek, Phillip Christopher, Mahdi M. Abu-Omar
|
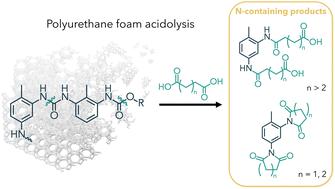
Obtaining circularity will be essential in managing plastic waste and moving towards sustainable materials. Chemical recycling offers a pathway to obtain valuable molecules from plastic waste, closing the loop on what is currently a linear economy. Here, we report on the chemical recycling of polyurethane foam (PUF) via acidolysis with dicarboxylic acids (DCAs) to release value-added molecules. While previous work has explored recovery of the recycled polyol (repolyol), we focus in this report on elucidating the product distribution and kinetics of the nitrogen-containing products from the acidolysis reaction. Using Nuclear Magnetic Resonance (NMR) spectroscopy and Ultra-High Pressure Liquid Chromatography – Mass Spectroscopy (UPLC-MS), we demonstrate how acid loading and structure influence product distribution of acidolysis. The use of excess acid can eliminate oligomeric content and aromatic amines from the product mixture. With DCAs composed of 2 or 3 carbons between the carboxylic acid groups, we observe the formation of imide products during acidolysis, which has only very recently been reported in the literature. Furthermore, the kinetics of imide formation were investigated and modeled for glutaric acid (GA) and succinic acid (SA), which form 6- and 5-membered cyclic imides, respectively. Di-imide formation with SA proceeds without accumulation of intermediates and is an order of magnitude faster than imide formation with GA, for which an amide-imide intermediate is detected and the rate of reaction is sensitive to steric hindrance. This report offers fundamental insights into the N-containing products formed during acidolysis, which will aid scale-up of closed-loop chemical recycling processes.
中文翻译:

聚氨酯泡沫与羧酸酸解:酸结构决定了含 N 产物的分布和动力学
获得循环性对于管理塑料垃圾和转向可持续材料至关重要。化学回收提供了一种从塑料垃圾中获取有价值的分子的途径,从而在目前的线性经济中形成闭环。在本文中,我们报道了聚氨酯泡沫 (PUF) 通过与二羧酸 (DCA) 酸解以释放增值分子的化学回收。虽然以前的工作已经探索了回收多元醇(再多元醇)的回收,但在本报告中,我们专注于阐明酸解反应中含氮产物的产物分布和动力学。使用核磁共振 (NMR) 光谱和超高压液相色谱-质谱 (UPLC-MS),我们展示了酸负荷和结构如何影响酸解的产物分布。使用过量的酸可以消除产品混合物中的低聚物含量和芳香胺。DCA 由羧酸基团之间的 2 或 3 个碳组成,我们观察到酸解过程中酰亚胺产物的形成,这最近才在文献中报道。此外,研究了戊二酸 (GA) 和琥珀酸 (SA) 的酰亚胺形成动力学,它们分别形成 6 元和 5 元环状酰亚胺。SA 的二酰亚胺形成过程没有中间体积累,比 GA 的酰亚胺形成快一个数量级,GA 检测到酰胺-酰亚胺中间体,反应速率对空间位阻敏感。本报告提供了对酸解过程中形成的含 N 产物的基本见解,这将有助于扩大闭环化学回收过程的规模。
更新日期:2024-11-12
中文翻译:

聚氨酯泡沫与羧酸酸解:酸结构决定了含 N 产物的分布和动力学
获得循环性对于管理塑料垃圾和转向可持续材料至关重要。化学回收提供了一种从塑料垃圾中获取有价值的分子的途径,从而在目前的线性经济中形成闭环。在本文中,我们报道了聚氨酯泡沫 (PUF) 通过与二羧酸 (DCA) 酸解以释放增值分子的化学回收。虽然以前的工作已经探索了回收多元醇(再多元醇)的回收,但在本报告中,我们专注于阐明酸解反应中含氮产物的产物分布和动力学。使用核磁共振 (NMR) 光谱和超高压液相色谱-质谱 (UPLC-MS),我们展示了酸负荷和结构如何影响酸解的产物分布。使用过量的酸可以消除产品混合物中的低聚物含量和芳香胺。DCA 由羧酸基团之间的 2 或 3 个碳组成,我们观察到酸解过程中酰亚胺产物的形成,这最近才在文献中报道。此外,研究了戊二酸 (GA) 和琥珀酸 (SA) 的酰亚胺形成动力学,它们分别形成 6 元和 5 元环状酰亚胺。SA 的二酰亚胺形成过程没有中间体积累,比 GA 的酰亚胺形成快一个数量级,GA 检测到酰胺-酰亚胺中间体,反应速率对空间位阻敏感。本报告提供了对酸解过程中形成的含 N 产物的基本见解,这将有助于扩大闭环化学回收过程的规模。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号