Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
pH controlled synthesis of end to end linked Au nanorod dimer in an aqueous solution for plasmon enhanced spectroscopic applications
Nanoscale ( IF 5.8 ) Pub Date : 2024-11-08 , DOI: 10.1039/d4nr03235g Shubhangi Sharma, Théo Minchella, Susmita Pradhan, Davy Gérard, Quanbo Jiang, Satyajit Patra
Nanoscale ( IF 5.8 ) Pub Date : 2024-11-08 , DOI: 10.1039/d4nr03235g Shubhangi Sharma, Théo Minchella, Susmita Pradhan, Davy Gérard, Quanbo Jiang, Satyajit Patra
|
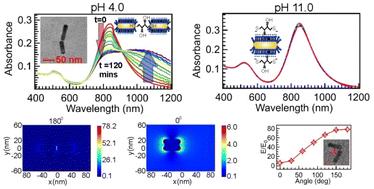
End-to-end linked nanorod dimer nanogap antennas exhibit superior plasmonic enhancement compared to monomers due to the coupling of localized surface plasmon resonances (LSPR) of individual nanorods. However, controlling the assembly to stop at the dimer stage is challenging. Here, we report a pH-controlled synthesis of Au nanorod dimer nanogap antennas in an aqueous solution using 1,4-dithiothreitol (DTT) as a linker. Neutral to acidic pH (4.0 to 7.0) favors dimer formation, while higher pH decreases dimer yield, stopping completely at pH 11.0. The reaction can also be halted in neutral and acidic solutions by abruptly increasing the pH to 11.0 or higher. At basic pH, both thiol groups of DTT deprotonate and acquire a negative charge, causing both thiolate ends to adsorb onto the positively charged cetyltrimethylammonium bromide (CTAB) micellar layer on the transverse surface of the Au nanorod, preventing dimer formation. TEM images confirm nanorod dimers, showing a good conversion yield (∼80%) from monomers to dimers. Overall, out of all the DTT induced NR assemblies, 70% are found to be dimers. The majority of these dimers (>90%) are end-to-end linked dimers, with a gap distance of ∼1 nm, exhibiting exceptional stability and remaining intact for over two weeks. FDTD simulations demonstrate a significant enhancement of the light E field in the nanogap, ∼80 times higher than in a homogeneous water environment and 11 times higher than in nanorod monomers. Simulations also show that E field enhancement varies with the angular separation of monomeric nanorods, being highest for end-to-end dimers (180°) and lower for side-to-side dimers (0°). Overall, we present an inexpensive method to design and control nanorod dimer nanogap antennas in aqueous solution, useful for plasmon-enhanced spectroscopic applications such as biosensing, chemical sensing, and biomedical devices.
中文翻译:

在水溶液中端对端连接的 Au 纳米棒二聚体的 pH 控制合成,用于等离激元增强光谱应用
由于单个纳米棒的局部表面等离子体共振 (LSPR) 的耦合,与单体相比,端到端连接的纳米棒二聚体纳米间隙天线表现出卓越的等离子体增强。然而,控制组装体在二聚体阶段停止是具有挑战性的。在这里,我们报道了使用 1,4-二硫苏糖醇 (DTT) 作为接头在水溶液中 pH 控制合成的 Au 纳米棒二聚体纳米间隙天线。中性至酸性 pH 值(4.0 至 7.0)有利于二聚体形成,而较高的 pH 值会降低二聚体产量,在 pH 值 11.0 时完全停止。在中性和酸性溶液中,也可以通过突然将 pH 值增加到 11.0 或更高来终止反应。在碱性 pH 值下,DTT 的两个巯基都去质子化并获得负电荷,导致硫代化物的两个末端吸附到 Au 纳米棒横向上带正电荷的十六烷基三甲基溴化铵 (CTAB) 胶束层上,从而阻止二聚体形成。TEM 图像证实了纳米棒二聚体,显示从单体到二聚体的良好转化率 (∼80%)。总体而言,在所有 DTT 诱导的 NR 组装体中,发现 70% 是二聚体。这些二聚体中的大多数 (>90%) 是端到端连接的二聚体,间隙距离为 ∼1 nm,表现出优异的稳定性,并保持完整状态超过两周。FDTD 模拟表明,纳米间隙中的光 E 场显著增强,比均质水环境中高 ∼80 倍,比纳米棒单体高 11 倍。模拟还表明,E 场增强随单体纳米棒的角度分离而变化,端到端二聚体 (180°) 最高,侧对侧二聚体 (0°) 较低。 总的来说,我们提出了一种在水溶液中设计和控制纳米棒二聚体纳米间隙天线的廉价方法,可用于等离子体增强光谱应用,如生物传感、化学传感和生物医学设备。
更新日期:2024-11-08
中文翻译:

在水溶液中端对端连接的 Au 纳米棒二聚体的 pH 控制合成,用于等离激元增强光谱应用
由于单个纳米棒的局部表面等离子体共振 (LSPR) 的耦合,与单体相比,端到端连接的纳米棒二聚体纳米间隙天线表现出卓越的等离子体增强。然而,控制组装体在二聚体阶段停止是具有挑战性的。在这里,我们报道了使用 1,4-二硫苏糖醇 (DTT) 作为接头在水溶液中 pH 控制合成的 Au 纳米棒二聚体纳米间隙天线。中性至酸性 pH 值(4.0 至 7.0)有利于二聚体形成,而较高的 pH 值会降低二聚体产量,在 pH 值 11.0 时完全停止。在中性和酸性溶液中,也可以通过突然将 pH 值增加到 11.0 或更高来终止反应。在碱性 pH 值下,DTT 的两个巯基都去质子化并获得负电荷,导致硫代化物的两个末端吸附到 Au 纳米棒横向上带正电荷的十六烷基三甲基溴化铵 (CTAB) 胶束层上,从而阻止二聚体形成。TEM 图像证实了纳米棒二聚体,显示从单体到二聚体的良好转化率 (∼80%)。总体而言,在所有 DTT 诱导的 NR 组装体中,发现 70% 是二聚体。这些二聚体中的大多数 (>90%) 是端到端连接的二聚体,间隙距离为 ∼1 nm,表现出优异的稳定性,并保持完整状态超过两周。FDTD 模拟表明,纳米间隙中的光 E 场显著增强,比均质水环境中高 ∼80 倍,比纳米棒单体高 11 倍。模拟还表明,E 场增强随单体纳米棒的角度分离而变化,端到端二聚体 (180°) 最高,侧对侧二聚体 (0°) 较低。 总的来说,我们提出了一种在水溶液中设计和控制纳米棒二聚体纳米间隙天线的廉价方法,可用于等离子体增强光谱应用,如生物传感、化学传感和生物医学设备。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号