当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure-Guided Design of Affinity/Covalent-Bond Dual-Driven Inhibitors Targeting the AMP Site of FBPase
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-11-09 , DOI: 10.1021/acs.jmedchem.4c01886 Hongxuan Cao, Zeyue Huang, Zheng Liu, Xiao Zhang, Yanliang Ren, Muhammad Salman Hameed, Li Rao, Nokwanda P. Makunga, Georgi M. Dobrikov, Jian Wan
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-11-09 , DOI: 10.1021/acs.jmedchem.4c01886 Hongxuan Cao, Zeyue Huang, Zheng Liu, Xiao Zhang, Yanliang Ren, Muhammad Salman Hameed, Li Rao, Nokwanda P. Makunga, Georgi M. Dobrikov, Jian Wan
|
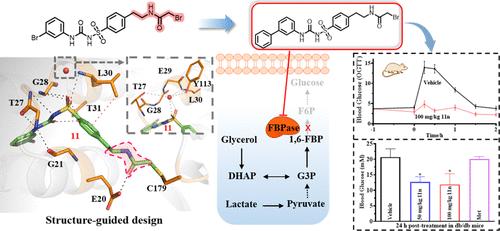
Fructose-1,6-bisphosphatase (FBPase) has attracted substantial interest as a target associated with cancer and type II diabetes. FBPase inhibitors targeting the AMP allosteric site have been documented, but their limited selectivity has raised concerns about adverse effects. To address this issue, we designed the affinity/covalent-bond dual-driven inhibitors based on the pharmacophore knowledge of the AMP pocket and neighboring cysteine residue (C179) of FBPase using the cysteine-targeting reactivity warhead screen followed by a structural optimization strategy. Pull-down and Western Blotting assays confirmed FBPase as a direct target in hepatic cells. X-ray cocrystallographic structure of FBPase-11 and Cov_DOX calculation demonstrated that hydrogen bonding and π–π stacking were the predominant driving force for the inhibition of sulfonylurea-based FBPase covalent inhibitors, while covalent binding with C179 enhances the inhibitors’ long-lasting hypoglycemic effects. Together, this work highlights the potential of affinity/covalent-bond dual-driven inhibitors in drug development and provides a promising approach for developing potent drugs targeting AMP-associated proteins.
中文翻译:

靶向 FBPase AMP 位点的亲和/共价键双驱动抑制剂的结构引导设计
果糖-1,6-二磷酸酶 (FBPase) 作为与癌症和 II 型糖尿病相关的靶点引起了人们的极大兴趣。靶向 AMP 变构位点的 FBP 酶抑制剂已有记录,但其有限的选择性引起了人们对不良反应的担忧。为了解决这个问题,我们根据 FBPase 的 AMP 口袋和邻近半胱氨酸残基 (C179) 的药效团知识,使用半胱氨酸靶向反应性弹头筛选,然后采用结构优化策略,设计了亲和/共价键双驱动抑制剂。Pull-down 和 Western Blotting 测定证实 FBP 酶是肝细胞中的直接靶标。FBPase-11 的 X 射线共晶结构和 Cov_DOX 计算表明,氢键和 π-π 堆积是抑制基于磺酰脲类的 FBPase 共价抑制剂的主要驱动力,而与 C179 的共价结合增强了抑制剂的长效降糖作用。总之,这项工作突出了亲和/共价键双驱动抑制剂在药物开发中的潜力,并为开发靶向 AMP 相关蛋白的强效药物提供了一种有前途的方法。
更新日期:2024-11-12
中文翻译:

靶向 FBPase AMP 位点的亲和/共价键双驱动抑制剂的结构引导设计
果糖-1,6-二磷酸酶 (FBPase) 作为与癌症和 II 型糖尿病相关的靶点引起了人们的极大兴趣。靶向 AMP 变构位点的 FBP 酶抑制剂已有记录,但其有限的选择性引起了人们对不良反应的担忧。为了解决这个问题,我们根据 FBPase 的 AMP 口袋和邻近半胱氨酸残基 (C179) 的药效团知识,使用半胱氨酸靶向反应性弹头筛选,然后采用结构优化策略,设计了亲和/共价键双驱动抑制剂。Pull-down 和 Western Blotting 测定证实 FBP 酶是肝细胞中的直接靶标。FBPase-11 的 X 射线共晶结构和 Cov_DOX 计算表明,氢键和 π-π 堆积是抑制基于磺酰脲类的 FBPase 共价抑制剂的主要驱动力,而与 C179 的共价结合增强了抑制剂的长效降糖作用。总之,这项工作突出了亲和/共价键双驱动抑制剂在药物开发中的潜力,并为开发靶向 AMP 相关蛋白的强效药物提供了一种有前途的方法。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号