当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient Adsorption of Multi-Heavy Metal Ions at Trace-Levels from Groundwater Using UiO-66 and Activated Carbon Nanocomposites for Safe Drinking
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-08 , DOI: 10.1021/acs.iecr.4c02399 Basit Ismail Gilani, Yogendra Nath Prajapati
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2024-11-08 , DOI: 10.1021/acs.iecr.4c02399 Basit Ismail Gilani, Yogendra Nath Prajapati
|
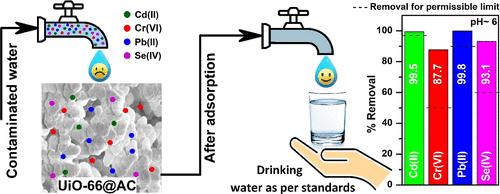
A composite of UiO-66 and activated carbon was synthesized by using a solvothermal method to efficiently remove heavy metal ions from synthetic water and groundwater at trace levels. The adsorbents were characterized for their physicochemical properties using FE-SEM, EDX, XRD, BET, TGA, FT-IR, and XPS. FE-SEM and EDX analyses confirmed the octahedral structure of nanoparticles, along with a homogeneous distribution of activated carbon on UiO-66. XRD patterns revealed that the samples were crystalline, with an average crystalline size of 39 nm. Under optimum operating conditions, the adsorbents exhibited maximum capacities for Cd(II), Cr(VI), Pb(II), and Se(IV) ions, determined to be 431.1, 351.8, 499.3, and 380.8 mg/g, respectively. In contrast, the adsorbent’s meaningful adsorption capacity against the permissible metal ion concentration in drinking water was calculated to be 1.12, 21.54, 25.26, and 37.94 mg/g. These values surpass those of the most similar MOFs and other porous materials. The thermodynamic study revealed that the adsorption of these metal ions was spontaneous and endothermic within the temperature range of 288.15–318.15 K. Notably, the presence of competitive coexisting ions had a minimal impact on the removal efficiency of heavy metal ions, emphasizing the selectivity of the adsorbent. The adsorbent demonstrated excellent performance in effectively removing metal ions from synthetic water and groundwater within the allowable limits for drinking water. The material showed good regeneration capability for up to 5 studied cycles, emphasizing the robust adsorption performance of the composite and highlighting its potential application in water treatment.
中文翻译:

使用 UiO-66 和活性炭纳米复合材料从地下水中高效吸附痕量多重金属离子,确保安全饮用
采用溶剂热法合成了 UiO-66 和活性炭的复合材料,可有效去除合成水和地下水中痕量级的重金属离子。使用 FE-SEM、EDX、XRD、BET、TGA、FT-IR 和 XPS 对吸附剂的物理化学性质进行了表征。FE-SEM 和 EDX 分析证实了纳米颗粒的八面体结构,以及活性炭在 UiO-66 上的均匀分布。XRD 图谱显示样品是结晶的,平均晶体尺寸为 39 nm。在最佳操作条件下,吸附剂对 Cd(II)、Cr(VI)、Pb(II) 和 Se(IV) 离子的最大容量分别为 431.1、351.8、499.3 和 380.8 mg/g。相比之下,吸附剂对饮用水中允许金属离子浓度的有效吸附能力计算为 1.12、21.54、25.26 和 37.94 mg/g。这些值超过了最相似的 MOF 和其他多孔材料的值。热力学研究表明,这些金属离子的吸附在 288.15–318.15 K 的温度范围内是自发的和吸热的。值得注意的是,竞争性共存离子的存在对重金属离子的去除效率影响最小,强调了吸附剂的选择性。该吸附剂在饮用水允许的范围内有效去除合成水和地下水中的金属离子方面表现出优异的性能。该材料在多达 5 个研究循环中显示出良好的再生能力,强调了复合材料的强大吸附性能,并突出了其在水处理中的潜在应用。
更新日期:2024-11-08
中文翻译:

使用 UiO-66 和活性炭纳米复合材料从地下水中高效吸附痕量多重金属离子,确保安全饮用
采用溶剂热法合成了 UiO-66 和活性炭的复合材料,可有效去除合成水和地下水中痕量级的重金属离子。使用 FE-SEM、EDX、XRD、BET、TGA、FT-IR 和 XPS 对吸附剂的物理化学性质进行了表征。FE-SEM 和 EDX 分析证实了纳米颗粒的八面体结构,以及活性炭在 UiO-66 上的均匀分布。XRD 图谱显示样品是结晶的,平均晶体尺寸为 39 nm。在最佳操作条件下,吸附剂对 Cd(II)、Cr(VI)、Pb(II) 和 Se(IV) 离子的最大容量分别为 431.1、351.8、499.3 和 380.8 mg/g。相比之下,吸附剂对饮用水中允许金属离子浓度的有效吸附能力计算为 1.12、21.54、25.26 和 37.94 mg/g。这些值超过了最相似的 MOF 和其他多孔材料的值。热力学研究表明,这些金属离子的吸附在 288.15–318.15 K 的温度范围内是自发的和吸热的。值得注意的是,竞争性共存离子的存在对重金属离子的去除效率影响最小,强调了吸附剂的选择性。该吸附剂在饮用水允许的范围内有效去除合成水和地下水中的金属离子方面表现出优异的性能。该材料在多达 5 个研究循环中显示出良好的再生能力,强调了复合材料的强大吸附性能,并突出了其在水处理中的潜在应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号