当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-Enzyme Catalyzed Stereoselective Synthesis of Chiral Aromatic Polysubstituted γ-Butyrolactones
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-08 , DOI: 10.1021/acscatal.4c04498 Liliang Chu, Xiaoyan Zhang, Daidi Fan, Yunpeng Bai
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-08 , DOI: 10.1021/acscatal.4c04498 Liliang Chu, Xiaoyan Zhang, Daidi Fan, Yunpeng Bai
|
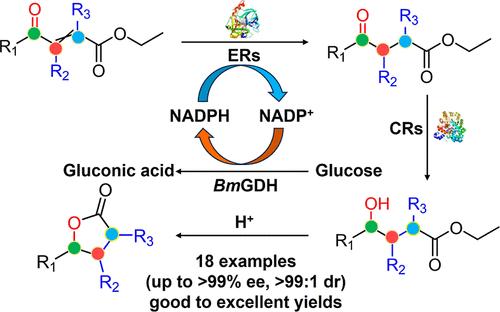
Chiral polysubstituted aromatic γ-butyrolactones are core structural units of many natural products and high value-added chemicals in the pharmaceutical and food industries. Currently, the precise construction of multiple chiral centers on the five-membered heterocycle substituted by bulky phenyl groups faces big challenges, such as low stereoselectivity, expensive noble metal catalysts, harsh reaction conditions and low atom economy. Herein, we report a one-pot, two-enzyme catalytic strategy for the synthesis of 18 bulky di/trisubstituted aromatic γ-butyrolactones on the α-, β- and γ-carbons with good enantioselectivities (up to >99% ee) and diastereoselectivities (up to >99:1 dr). This cascade process includes sequential two-step asymmetric reduction of α-/β-unsaturated γ-ketoesters by four ene reductases and a carbonyl reductase without intermediate isolation and catalyst removal. In particular, the large sterically hindered substrates (1p–1s) were converted to the corresponding trisubstituted γ-butyrolactones (4p–4s) with 98–99% ee and >99:1 dr. This enzymatic cascade process represents a simple, atom-economic and enantioselective method to deliver a broad of bulky polysubstituted γ-butyrolactones in a cheap and efficient manner compared to conventional methods.
中文翻译:

双酶催化立体选择性合成手性芳香族多取代γ-丁内酯
手性多取代芳香族 γ-丁内酯是制药和食品工业中许多天然产物和高附加值化学品的核心结构单元。目前,在大体积苯基取代的五元杂环上精确构建多个手性中心面临着巨大的挑战,例如低立体选择性、昂贵的贵金属催化剂、苛刻的反应条件和低原子经济性。在此,我们报道了一种一锅法、双酶催化策略,用于在 α-、β-和 γ-碳上合成 18 个大体积的二/三取代芳香族 γ-丁内酯,具有良好的对映选择性(高达 >99% ee)和非对映选择性(高达 >99:1 dr)。该级联过程包括四种烯还原酶和一种羰基还原酶对 α-/β-不饱和γ-酮酯进行连续两步不对称还原,无需中间分离和催化剂去除。特别是,将大的空间位阻底物 (1p-1s) 转化为相应的具有 98-99% ee 和 >99:1 dr 的三取代 γ-丁内酯 (4p-4s)。这种酶促级联反应过程代表了一种简单的、原子经济的对映选择性方法,与传统方法相比,它以廉价和有效的方式递送大量大体积的多取代γ-丁内酯。
更新日期:2024-11-08
中文翻译:

双酶催化立体选择性合成手性芳香族多取代γ-丁内酯
手性多取代芳香族 γ-丁内酯是制药和食品工业中许多天然产物和高附加值化学品的核心结构单元。目前,在大体积苯基取代的五元杂环上精确构建多个手性中心面临着巨大的挑战,例如低立体选择性、昂贵的贵金属催化剂、苛刻的反应条件和低原子经济性。在此,我们报道了一种一锅法、双酶催化策略,用于在 α-、β-和 γ-碳上合成 18 个大体积的二/三取代芳香族 γ-丁内酯,具有良好的对映选择性(高达 >99% ee)和非对映选择性(高达 >99:1 dr)。该级联过程包括四种烯还原酶和一种羰基还原酶对 α-/β-不饱和γ-酮酯进行连续两步不对称还原,无需中间分离和催化剂去除。特别是,将大的空间位阻底物 (1p-1s) 转化为相应的具有 98-99% ee 和 >99:1 dr 的三取代 γ-丁内酯 (4p-4s)。这种酶促级联反应过程代表了一种简单的、原子经济的对映选择性方法,与传统方法相比,它以廉价和有效的方式递送大量大体积的多取代γ-丁内酯。

































 京公网安备 11010802027423号
京公网安备 11010802027423号