当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
H2-Evolving Cobalt–Protic-NHC Catalysts: Kinetic Zone Diagram Analysis and Mechanistic Insights
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-08 , DOI: 10.1021/acscatal.4c05104 Sanajit Kumar Mandal, Anusha Gupta, Joyanta Choudhury
ACS Catalysis ( IF 11.3 ) Pub Date : 2024-11-08 , DOI: 10.1021/acscatal.4c05104 Sanajit Kumar Mandal, Anusha Gupta, Joyanta Choudhury
|
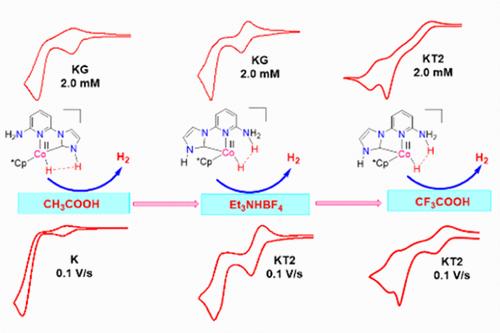
A series of systematically designed cobalt–protic-NHC complexes containing pendant proton-shuttle groups was synthesized. The proton-shuttle motifs enabled these complexes to act as efficient electrocatalysts for the hydrogen evolution reaction (HER) from various acids as proton sources. The effect of acid strength on the mechanism of HER was investigated by varying the proton source ( CH3COOH, pKaCH3CN = 23.51), triethylammonium tetrafluoroborate (Et3NHBF4, pKaCH3CN = 18.57), and trifluoroacetic acid (CF3COOH, pKaCH3CN = 12.70). Additionally, by changing experimental parameters such as substrate/catalyst concentration and scan rate, the single-electron EC′ zone diagram could be extended to the present multielectron reaction system where all of the zones were accessed with little deviation in some of the waveforms from the original. From the kinetic zone diagram analysis, some of the performance parameters such as the observed rate constant (kobs), turnover frequency (TOF), and the rate constant of the first chemical step (k1) were determined. Also, the zone diagram provided insight into the mechanistic cycle and the nature of the rate-limiting step. The investigation suggested that the protic proton of the proton-shuttle functionality triggered a hydrogen evolution reaction via intramolecular proton-hydride coupling from the Co(II)–H intermediate. This intramolecular dihydrogen elimination step, which was independent of the acid concentration, acted as the rate-limiting step and the turnover frequency of HER was fully controlled by this step.
中文翻译:

H2 - 不断发展的钴-质子-NHC 催化剂:动力学区图分析和机理见解
合成了一系列系统设计的钴-质子-NHC 配合物,其中包含悬垂质子穿梭基团。质子穿梭基序使这些复合物能够作为来自各种酸作为质子源的析氢反应 (HER) 的有效电催化剂。通过改变质子源 ( CH3COOH, pKaCH3CN = 23.51)、四氟硼酸三乙基铵 (Et3NHBF4, pKaCH3CN = 18.57) 和三氟乙酸 (CF3COOH, pKaCH3CN ) 来研究酸强度对 HER 机制的影响= 12.70)。此外,通过改变实验参数,如底物/催化剂浓度和扫描速率,单电子 EC′ 区图可以扩展到目前的多电子反应系统,其中所有区都可以访问,并且某些波形与原始波形几乎没有偏差。从动力学区图分析中,确定了一些性能参数,例如观察到的速率常数 (kobs)、周转频率 (TOF) 和第一个化学步骤的速率常数 (k1)。此外,区域图还提供了对机制循环和限速步骤性质的见解。研究表明,质子穿梭功能的质子通过来自 Co(II)-H 中间体的分子内质子-氢化物偶联触发了析氢反应。这个与酸浓度无关的分子内二氢消除步骤充当限速步骤,并且 HER 的周转频率完全由该步骤控制。
更新日期:2024-11-08
中文翻译:

H2 - 不断发展的钴-质子-NHC 催化剂:动力学区图分析和机理见解
合成了一系列系统设计的钴-质子-NHC 配合物,其中包含悬垂质子穿梭基团。质子穿梭基序使这些复合物能够作为来自各种酸作为质子源的析氢反应 (HER) 的有效电催化剂。通过改变质子源 ( CH3COOH, pKaCH3CN = 23.51)、四氟硼酸三乙基铵 (Et3NHBF4, pKaCH3CN = 18.57) 和三氟乙酸 (CF3COOH, pKaCH3CN ) 来研究酸强度对 HER 机制的影响= 12.70)。此外,通过改变实验参数,如底物/催化剂浓度和扫描速率,单电子 EC′ 区图可以扩展到目前的多电子反应系统,其中所有区都可以访问,并且某些波形与原始波形几乎没有偏差。从动力学区图分析中,确定了一些性能参数,例如观察到的速率常数 (kobs)、周转频率 (TOF) 和第一个化学步骤的速率常数 (k1)。此外,区域图还提供了对机制循环和限速步骤性质的见解。研究表明,质子穿梭功能的质子通过来自 Co(II)-H 中间体的分子内质子-氢化物偶联触发了析氢反应。这个与酸浓度无关的分子内二氢消除步骤充当限速步骤,并且 HER 的周转频率完全由该步骤控制。

































 京公网安备 11010802027423号
京公网安备 11010802027423号