当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of Metal-Oxide Interfaces in Methanol Decomposition: Reaction of Methanol on CeO2/Ag(111) Inverse Model Catalysts
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.jpclett.4c02878 Dongling Zhang, Xu Cao, Xingwang Cheng, Luchao Huang, Yi Tu, Honghe Ding, Jun Hu, Qian Xu, Junfa Zhu
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.jpclett.4c02878 Dongling Zhang, Xu Cao, Xingwang Cheng, Luchao Huang, Yi Tu, Honghe Ding, Jun Hu, Qian Xu, Junfa Zhu
|
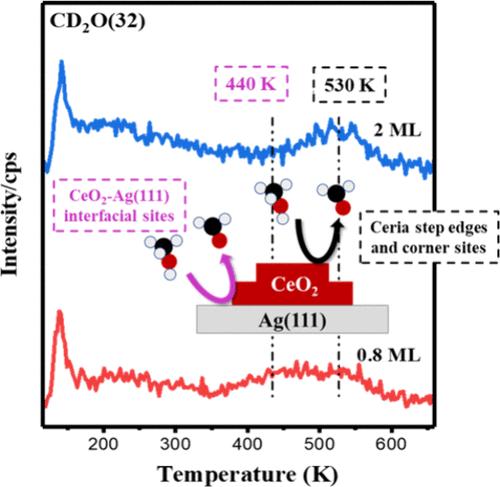
Metal-oxide interfaces play a critical role in catalytic processes, such as methanol adsorption and decomposition reactions. In this work, we investigated methanol reactions on the inverse model CeO2/Ag(111) catalyst surfaces, i.e., submonolayer CeO2 films on Ag(111), under ultrahigh vacuum (UHV) conditions to specially address the role of CeO2–Ag interface in the catalytic methanol decomposition reactions. Using scanning tunneling microscopy (STM), low-energy electron diffraction (LEED), and synchrotron radiation photoemission spectroscopy (SRPES), we found that, at the submonolayer ceria coverages, the CeO2 nanoislands exhibit a hexagonal CeO2(111) lattice with fully oxidized Ce4+ on Ag(111). At higher ceria coverages, multilayer ceria nanoislands form on the Ag(111) surface instead of a well-ordered film. A combination of temperature-programmed desorption (TPD) and SRPES reveals that methanol adsorbs dissociatively on the CeO2/Ag(111) surfaces at 110 K, resulting in the formation of methoxy groups. These methoxy groups subsequently decompose via two pathways: (i) interaction with lattice oxygen to produce formate species at 230 K, which then decompose to CO, and (ii) direct dehydrogenation of methoxy to formaldehyde. Notably, the surface with submonolayer CeO2 film on Ag(111) demonstrates low-temperature reactivity (440 K) for methoxy dehydrogenation to formaldehyde, which occurs at a much lower temperature, compared to the surface of multilayer CeO2 on Ag(111) surface (530 K). This finding emphasizes that the CeO2–Ag(111) interfaces provide unique active sites for methoxy dehydrogenation reactions.
中文翻译:

金属氧化物界面在甲醇分解中的作用:甲醇与 CeO2/Ag(111) 逆模型催化剂的反应
金属氧化物界面在催化过程中起着关键作用,例如甲醇吸附和分解反应。在这项工作中,我们研究了在超高真空 (UHV) 条件下逆模型 CeO2/Ag(111) 催化剂表面(即 Ag(111) 上的亚单层 CeO2 膜上的甲醇反应,以专门解决 CeO2-Ag 界面在催化甲醇分解反应中的作用。使用扫描隧道显微镜 (STM)、低能电子衍射 (LEED) 和同步辐射光电子能谱 (SRPES),我们发现,在亚单层铈覆盖处,CeO2 纳米岛表现出六方 CeO2(111) 晶格,在 Ag(111) 上具有完全氧化的 Ce4+。在较高的铈覆盖率下,多层铈纳米岛在 Ag(111) 表面形成,而不是有序的薄膜。程序升温解吸 (TPD) 和 SRPES 的组合表明,甲醇在 110 K 时解离吸附在 CeO2/Ag(111) 表面上,导致形成甲氧基。这些甲氧基随后通过两种途径分解:(i) 与 晶格氧 相互作用在 230 K 时产生甲酸盐物种,然后分解成 CO,以及 (ii) 甲氧基直接脱氢生成甲醛。值得注意的是,与 Ag(111) 表面 (530 K) 上的多层 CeO 2 表面相比,Ag(111) 上具有亚单层 CeO2 膜的表面表现出甲氧基脱氢成甲醛的低温反应性 (440 K),这与 Ag(111) 表面的多层 CeO2 表面相比,发生在低得多的温度下。这一发现强调了 CeO2-Ag(111) 界面为甲氧基脱氢反应提供了独特的活性位点。
更新日期:2024-11-07
中文翻译:

金属氧化物界面在甲醇分解中的作用:甲醇与 CeO2/Ag(111) 逆模型催化剂的反应
金属氧化物界面在催化过程中起着关键作用,例如甲醇吸附和分解反应。在这项工作中,我们研究了在超高真空 (UHV) 条件下逆模型 CeO2/Ag(111) 催化剂表面(即 Ag(111) 上的亚单层 CeO2 膜上的甲醇反应,以专门解决 CeO2-Ag 界面在催化甲醇分解反应中的作用。使用扫描隧道显微镜 (STM)、低能电子衍射 (LEED) 和同步辐射光电子能谱 (SRPES),我们发现,在亚单层铈覆盖处,CeO2 纳米岛表现出六方 CeO2(111) 晶格,在 Ag(111) 上具有完全氧化的 Ce4+。在较高的铈覆盖率下,多层铈纳米岛在 Ag(111) 表面形成,而不是有序的薄膜。程序升温解吸 (TPD) 和 SRPES 的组合表明,甲醇在 110 K 时解离吸附在 CeO2/Ag(111) 表面上,导致形成甲氧基。这些甲氧基随后通过两种途径分解:(i) 与 晶格氧 相互作用在 230 K 时产生甲酸盐物种,然后分解成 CO,以及 (ii) 甲氧基直接脱氢生成甲醛。值得注意的是,与 Ag(111) 表面 (530 K) 上的多层 CeO 2 表面相比,Ag(111) 上具有亚单层 CeO2 膜的表面表现出甲氧基脱氢成甲醛的低温反应性 (440 K),这与 Ag(111) 表面的多层 CeO2 表面相比,发生在低得多的温度下。这一发现强调了 CeO2-Ag(111) 界面为甲氧基脱氢反应提供了独特的活性位点。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号