当前位置:
X-MOL 学术
›
Polym. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Sequence-defined structural transitions by calcium-responsive proteins
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-11-08 , DOI: 10.1039/d4py00907j Marina P. Chang, Winnie Huang, Gatha M. Shambharkar, Kenny M. Hernandez, Danielle J. Mai
Polymer Chemistry ( IF 4.1 ) Pub Date : 2024-11-08 , DOI: 10.1039/d4py00907j Marina P. Chang, Winnie Huang, Gatha M. Shambharkar, Kenny M. Hernandez, Danielle J. Mai
|
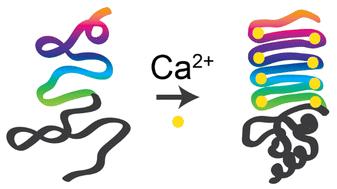
Biopolymer sequences dictate their functions, and protein-based polymers are a promising platform to establish sequence–function relationships for novel biopolymers. To efficiently explore vast sequence spaces of natural proteins, sequence repetition is a common strategy to tune and amplify specific functions. This strategy is applied to repeats-in-toxin (RTX) proteins with calcium-responsive folding behavior, which stems from tandem repeats of the nonapeptide GGXGXDXUX in which X can be any amino acid and U is a hydrophobic amino acid. To determine the functional range of this nonapeptide, we modified a naturally occurring RTX protein that forms β-roll structures in the presence of calcium. Sequence modifications focused on calcium-binding turns within the repetitive region, including either global substitution of nonconserved residues or complete replacement with tandem repeats of a consensus nonapeptide GGAGXDTLY. Some sequence modifications disrupted the typical transition from intrinsically disordered random coils to folded β rolls, despite conservation of the underlying nonapeptide sequence. Proteins enriched with smaller, hydrophobic amino acids adopted secondary structures in the absence of calcium and underwent structural rearrangements in calcium-rich environments. In contrast, proteins with bulkier, hydrophilic amino acids maintained intrinsic disorder in the absence of calcium. These results indicate a significant role of nonconserved amino acids in calcium-responsive folding, thereby revealing a strategy to leverage sequences in the design of tunable, calcium-responsive biopolymers.
中文翻译:

钙反应蛋白的序列定义的结构转变
生物聚合物序列决定了它们的功能,而基于蛋白质的聚合物是建立新型生物聚合物序列-功能关系的一个有前途的平台。为了有效地探索天然蛋白质的庞大序列空间,序列重复是调整和扩增特定功能的常用策略。该策略适用于具有钙反应性折叠行为的毒素重复序列 (RTX) 蛋白,这源于九肽 GGXGXDXUX 的串联重复序列,其中 X 可以是任何氨基酸,U 是疏水性氨基酸。为了确定这种九肽的功能范围,我们修饰了一种天然存在的 RTX 蛋白,该蛋白在钙存在下形成β卷结构。序列修饰集中在重复区域内的钙结合转折上,包括非保守残基的全局替换或完全替换为共有九肽 GGAGXDTLY 的串联重复序列。尽管潜在的九肽序列是保守的,但一些序列修饰破坏了从固有无序随机线圈到折叠β卷的典型转变。富含较小疏水氨基酸的蛋白质在缺乏钙的情况下采用二级结构,并在富含钙的环境中发生结构重排。相比之下,具有体积较大的亲水性氨基酸的蛋白质在缺乏钙的情况下保持了内在无序。这些结果表明非保守氨基酸在钙响应性折叠中起重要作用,从而揭示了一种在设计可调谐、钙响应性生物聚合物中利用序列的策略。
更新日期:2024-11-08
中文翻译:

钙反应蛋白的序列定义的结构转变
生物聚合物序列决定了它们的功能,而基于蛋白质的聚合物是建立新型生物聚合物序列-功能关系的一个有前途的平台。为了有效地探索天然蛋白质的庞大序列空间,序列重复是调整和扩增特定功能的常用策略。该策略适用于具有钙反应性折叠行为的毒素重复序列 (RTX) 蛋白,这源于九肽 GGXGXDXUX 的串联重复序列,其中 X 可以是任何氨基酸,U 是疏水性氨基酸。为了确定这种九肽的功能范围,我们修饰了一种天然存在的 RTX 蛋白,该蛋白在钙存在下形成β卷结构。序列修饰集中在重复区域内的钙结合转折上,包括非保守残基的全局替换或完全替换为共有九肽 GGAGXDTLY 的串联重复序列。尽管潜在的九肽序列是保守的,但一些序列修饰破坏了从固有无序随机线圈到折叠β卷的典型转变。富含较小疏水氨基酸的蛋白质在缺乏钙的情况下采用二级结构,并在富含钙的环境中发生结构重排。相比之下,具有体积较大的亲水性氨基酸的蛋白质在缺乏钙的情况下保持了内在无序。这些结果表明非保守氨基酸在钙响应性折叠中起重要作用,从而揭示了一种在设计可调谐、钙响应性生物聚合物中利用序列的策略。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号