当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Effects of surface oxygen vacancy on CO2 adsorption and its activation towards C2H4 using metal (Cu, Pd, CuPd) cluster-loaded TiO2 catalysts: a first principles study
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-08 , DOI: 10.1039/d4cp03507k Sajjad Hussain, Lina Zhang, Zhengzheng Xie, Jianjun Yang, Qiuye Li
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-11-08 , DOI: 10.1039/d4cp03507k Sajjad Hussain, Lina Zhang, Zhengzheng Xie, Jianjun Yang, Qiuye Li
|
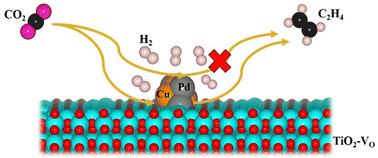
The conversion of the highly selective CO2 reduction reaction (CO2RR) into desired value-added multicarbon compounds, like C2H4, is crucial, but it is mainly constrained by the high energy barrier for C–C coupling and the multi-electron transfer process. Herein, M/TiO2 and M/TiO2–VO (M = Cu, Pd, CuPd, and VO refers to the surface oxygen vacancy) catalysts were designed to study the CO2RR towards C2H4 by using density functional theory (DFT). We found that the surface oxygen vacancy enhances the adsorption ability of studied catalysts. The CO2 molecule is strongly adsorbed at the metal–surface interfaces of Cu/TiO2–VO, Pd/TiO2–VO and CuPd/TiO2–VO catalysts with adsorption energies of −1.79, −1.75 and −1.71 eV, respectively. Furthermore, the C–C coupling reaction does not occur on the Cu and PdCu cluster sites of the M/TiO2–VO catalysts, indicating the inactivity of these sites for C2 products. However, Pd/TiO2, CuPd/TiO2 and M/TiO2–VO interfaces favor the C–C coupling reaction and therefore have the potential to reduce CO2 to C2 products. Additionally, the Gibbs free energy calculations reveal that the surface oxygen vacancy improves the OCCO hydrogenation to C2H4 at the CuPd/TiO2–VO interface.
中文翻译:

表面氧空位对金属(Cu、Pd、CuPd)负载 TiO2 催化剂对 CO2 吸附及其对 C2H4 活化的影响:第一性原理研究
将高选择性 CO2 还原反应 (CO2RR) 转化为所需的增值多碳化合物(如 C2H4)至关重要,但它主要受到 C-C 耦合和多电子转移过程的高能垒的限制。本文设计了 M/TiO2 和 M/TiO2–VO (M = Cu、Pd、CuPd 和 VO 是指表面氧空位)催化剂,利用密度泛函理论 (DFT) 研究了 CO2RR 朝向 C2H4 的变化。我们发现表面氧空位增强了所研究催化剂的吸附能力。CO2 分子强烈吸附在 Cu/TiO2–VO、Pd/TiO2–VO 和 CuPd/TiO2–VO 催化剂的金属表面界面上,吸附能分别为 -1.79、-1.75 和 -1.71 eV。此外,C-C 偶联反应不会发生在 M/TiO2-V O 催化剂的 Cu 和 PdCu 簇位点上,这表明这些位点对 C2 产物无活性。然而,Pd/TiO2、CuPd/TiO2 和 M/TiO2–VO 界面有利于 C-C 偶联反应,因此有可能将 CO2 还原为 C2 产物。 此外,吉布斯自由能计算表明,表面氧空位在 CuPd/TiO2–VO 界面处将 OCCO 氢化提高到 C2H4。
更新日期:2024-11-08
中文翻译:

表面氧空位对金属(Cu、Pd、CuPd)负载 TiO2 催化剂对 CO2 吸附及其对 C2H4 活化的影响:第一性原理研究
将高选择性 CO2 还原反应 (CO2RR) 转化为所需的增值多碳化合物(如 C2H4)至关重要,但它主要受到 C-C 耦合和多电子转移过程的高能垒的限制。本文设计了 M/TiO2 和 M/TiO2–VO (M = Cu、Pd、CuPd 和 VO 是指表面氧空位)催化剂,利用密度泛函理论 (DFT) 研究了 CO2RR 朝向 C2H4 的变化。我们发现表面氧空位增强了所研究催化剂的吸附能力。CO2 分子强烈吸附在 Cu/TiO2–VO、Pd/TiO2–VO 和 CuPd/TiO2–VO 催化剂的金属表面界面上,吸附能分别为 -1.79、-1.75 和 -1.71 eV。此外,C-C 偶联反应不会发生在 M/TiO2-V O 催化剂的 Cu 和 PdCu 簇位点上,这表明这些位点对 C2 产物无活性。然而,Pd/TiO2、CuPd/TiO2 和 M/TiO2–VO 界面有利于 C-C 偶联反应,因此有可能将 CO2 还原为 C2 产物。 此外,吉布斯自由能计算表明,表面氧空位在 CuPd/TiO2–VO 界面处将 OCCO 氢化提高到 C2H4。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号