当前位置:
X-MOL 学术
›
J. Phys. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing Aromaticity with Supersonic Jet Spectroscopy: A Case Study on Furan, Thiophene, and Selenophene
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.jpclett.4c02791 Akshay Kumar Sahu, Anant Ram Satpathi, Saiprakash Rout, Pranay Mohanty, Laxmipriya Dash, Himansu S. Biswal
The Journal of Physical Chemistry Letters ( IF 4.8 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.jpclett.4c02791 Akshay Kumar Sahu, Anant Ram Satpathi, Saiprakash Rout, Pranay Mohanty, Laxmipriya Dash, Himansu S. Biswal
|
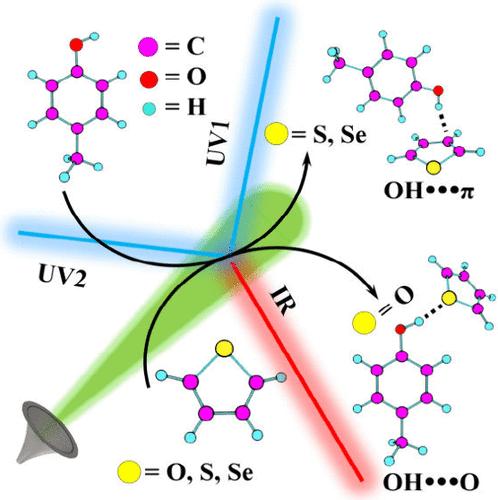
Aromaticity is a century-old concept that is even introduced in high school textbooks. However, the determination of the order of aromaticity of molecules as simple as furan, thiophene, and selenophene is still challenging. This work describes how different theoretical and experimental methods posit different aromaticity orders. To benchmark the theoretical results and arrive at a conclusion, mass-selective electronic and vibrational spectroscopy of these five-membered heterocycles under isolated supersonic-jet-cooled conditions was necessary. Since the aromaticity order can be unveiled from the magnitude of the electron density in the ring, we used hydrogen bonding as a probe. The experimental results revealed that selenophene forms the strongest π-hydrogen bond, suggesting that selenophene is the most aromatic, followed by thiophene and furan. It is concluded that gauge-including magnetically induced currents (GIMIC) and relative 1H and 13C NMR chemical shifts are better parameters to determine the aromaticity order in a similar class of molecules.
中文翻译:

用超音速射流光谱探测芳香性:以呋喃、噻吩和硒烯为例
芳香性是一个有百年历史的概念,甚至在高中教科书中也有介绍。然而,确定呋喃、噻吩和硒酚等简单分子的芳香性顺序仍然具有挑战性。这项工作描述了不同的理论和实验方法如何假设不同的芳香性阶数。为了对理论结果进行基准测试并得出结论,有必要在孤立的超音速喷射冷却条件下对这五元杂环进行质量选择性电子和振动光谱分析。由于芳香性阶数可以从环中电子密度的大小来揭示,因此我们使用氢键作为探针。实验结果表明,硒酚形成最强的π氢键,表明硒酚是最芳香的,其次是噻吩和呋喃。得出的结论是,包括磁感应电流 (GIMIC) 和相对 1H 和 13C NMR 化学位移是确定类似类别分子中芳香性顺序的更好参数。
更新日期:2024-11-07
中文翻译:

用超音速射流光谱探测芳香性:以呋喃、噻吩和硒烯为例
芳香性是一个有百年历史的概念,甚至在高中教科书中也有介绍。然而,确定呋喃、噻吩和硒酚等简单分子的芳香性顺序仍然具有挑战性。这项工作描述了不同的理论和实验方法如何假设不同的芳香性阶数。为了对理论结果进行基准测试并得出结论,有必要在孤立的超音速喷射冷却条件下对这五元杂环进行质量选择性电子和振动光谱分析。由于芳香性阶数可以从环中电子密度的大小来揭示,因此我们使用氢键作为探针。实验结果表明,硒酚形成最强的π氢键,表明硒酚是最芳香的,其次是噻吩和呋喃。得出的结论是,包括磁感应电流 (GIMIC) 和相对 1H 和 13C NMR 化学位移是确定类似类别分子中芳香性顺序的更好参数。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号