Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Observing root growth and signalling responses to stress gradients and pathogens using the bi-directional dual-flow RootChip
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-11-07 , DOI: 10.1039/d4lc00659c Claudia Allan, Yiling Sun, Stephen C. Whisson, Michael Porter, Petra C. Boevink, Volker Nock, Claudia-Nicole Meisrimler
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-11-07 , DOI: 10.1039/d4lc00659c Claudia Allan, Yiling Sun, Stephen C. Whisson, Michael Porter, Petra C. Boevink, Volker Nock, Claudia-Nicole Meisrimler
|
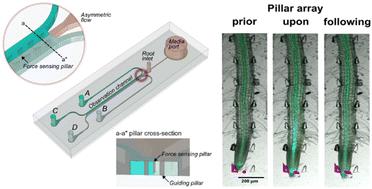
Plants respond to environmental stressors with adaptive changes in growth and development. Central to these responses is the role of calcium (Ca2+) as a key secondary messenger. Here, the bi-directional dual-flow RootChip (bi-dfRC) microfluidic platform was used to study defence signalling and root growth. By introducing salinity as sodium chloride (NaCl) treatment via a multiplexed media delivery system (MMDS), dynamic gradients were created, mimicking natural environmental fluctuations. Signal analysis in Arabidopsis thaliana plants showed that the Ca2+ burst indicated by the G-CaMP3 was concentration dependent. A Ca2+ burst initiated in response to salinity increase, specifically within the stele tissue, for 30 seconds. The signal then intensified in epidermal cells directly in contact with the stressor, spreading directionally towards the root tip, over 5 minutes. Inhibition of propidium iodide (PI) stain transport through the xylem was observed following salinity increase, contrasting with flow observed under control conditions. The interaction of Phytophthora capsici zoospores with A. thaliana roots was also studied. An immediate directional Ca2+ signal was observed during early pathogen recognition, while a gradual, non-directional increase was observed in Orp1_roGFP fluorescent H2O2 levels, over 30 min. By adjusting the dimensions of the bi-dfRC, plants with varying root architectures were subjected to growth analysis. Growth reduction was observed in A. thaliana and Nicotiana benthamiana roots when exposed to salinity induced by 100 mM NaCl, while Solanum lycopersicum exhibited growth increase over 90 minutes at the same NaCl concentration. Furthermore, novel insights into force sensing in roots were gained through the engineering of displaceable pillars into the bi-dfRC channel. These findings highlight the vital role of controlling fluid flow in microfluidic channels in advancing our understanding of root physiology under stress conditions.
中文翻译:

使用双向双流 RootChip 观察根系生长和对胁迫梯度和病原体的信号反应
植物通过生长和发育的适应性变化来响应环境压力。这些反应的核心是钙 (Ca2+) 作为关键二级信使的作用。在这里,双向双流 RootChip (bi-dfRC) 微流控平台用于研究防御信号和根生长。通过多重介质输送系统 (MMDS) 将盐度作为氯化钠 (NaCl) 处理引入,从而产生动态梯度,模拟自然环境波动。拟南芥植物的信号分析表明,G-CaMP3 指示的 Ca2+ 爆发是浓度依赖性的。Ca2+ 爆发响应盐度增加而启动,特别是在石碑组织内,持续 30 秒。然后,信号在直接与压力源接触的表皮细胞中增强,在 5 分钟内向根尖方向传播。盐度增加后观察到碘化丙啶 (PI) 染色剂通过木质部的运输受到抑制,这与在对照条件下观察到的流量形成鲜明对比。还研究了辣椒疫霉游动孢子与拟南芥根的相互作用。在早期病原体识别过程中观察到直接定向的 Ca2+ 信号,而在 30 分钟内观察到Orp1_roGFP荧光 H2O2 水平逐渐、无方向性增加。通过调整 bi-dfRC 的尺寸,对具有不同根结构的植物进行生长分析。在 A 中观察到生长减少。 当暴露于 100 mM NaCl 诱导的盐度时,拟南芥和本氏烟草根,而在相同的 NaCl 浓度下,Solanum lycopersicum 在 90 分钟内表现出生长增加。此外,通过将可移动柱工程化到 bi-dfRC 通道中,获得了对根中力传感的新见解。这些发现强调了控制微流体通道中的流体流动在促进我们对压力条件下根系生理学的理解方面的重要作用。
更新日期:2024-11-07
中文翻译:

使用双向双流 RootChip 观察根系生长和对胁迫梯度和病原体的信号反应
植物通过生长和发育的适应性变化来响应环境压力。这些反应的核心是钙 (Ca2+) 作为关键二级信使的作用。在这里,双向双流 RootChip (bi-dfRC) 微流控平台用于研究防御信号和根生长。通过多重介质输送系统 (MMDS) 将盐度作为氯化钠 (NaCl) 处理引入,从而产生动态梯度,模拟自然环境波动。拟南芥植物的信号分析表明,G-CaMP3 指示的 Ca2+ 爆发是浓度依赖性的。Ca2+ 爆发响应盐度增加而启动,特别是在石碑组织内,持续 30 秒。然后,信号在直接与压力源接触的表皮细胞中增强,在 5 分钟内向根尖方向传播。盐度增加后观察到碘化丙啶 (PI) 染色剂通过木质部的运输受到抑制,这与在对照条件下观察到的流量形成鲜明对比。还研究了辣椒疫霉游动孢子与拟南芥根的相互作用。在早期病原体识别过程中观察到直接定向的 Ca2+ 信号,而在 30 分钟内观察到Orp1_roGFP荧光 H2O2 水平逐渐、无方向性增加。通过调整 bi-dfRC 的尺寸,对具有不同根结构的植物进行生长分析。在 A 中观察到生长减少。 当暴露于 100 mM NaCl 诱导的盐度时,拟南芥和本氏烟草根,而在相同的 NaCl 浓度下,Solanum lycopersicum 在 90 分钟内表现出生长增加。此外,通过将可移动柱工程化到 bi-dfRC 通道中,获得了对根中力传感的新见解。这些发现强调了控制微流体通道中的流体流动在促进我们对压力条件下根系生理学的理解方面的重要作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号