Diabetologia ( IF 8.4 ) Pub Date : 2024-11-07 , DOI: 10.1007/s00125-024-06301-6 Pascal Gottmann, Thilo Speckmann, Mandy Stadion, Prateek Chawla, Judith Saurenbach, Nikolay Ninov, Heiko Lickert, Annette Schürmann
|
Aims/hypothesis
The aim of this work was to understand the role of non-beta cells in pancreatic islets at early stages of type 2 diabetes pathogenesis.
Methods
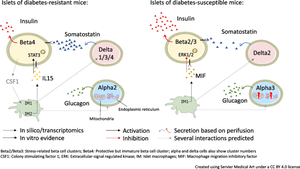
Specific clustering was employed to single-cell transcriptome data from islet cells of obese mouse strains differing in their diabetes susceptibility (diabetes-resistant B6.V.Lepob/ob [OB] and diabetes-susceptible New Zealand Obese [NZO] mice) on a diabetogenic diet.
Results
Refined clustering analysis revealed several heterogeneous subpopulations for alpha cells, delta cells and macrophages, of which 133 mapped to human diabetes genes identified by genome-wide association studies. Importantly, a similar non-beta cell heterogeneity was found in a dataset of human islets from donors at different stages of type 2 diabetes. The predominant alpha cell cluster in NZO mice displayed signs of cellular stress and lower mitochondrial capacity (97 differentially expressed genes [DEGs]), whereas delta cells from these mice exhibited higher expression levels of maturation marker genes (Hhex and Sst) but lower somatostatin secretion than OB mice (184 DEGs). Furthermore, a cluster of macrophages was almost twice as abundant in islets of OB mice, and displayed extensive cell–cell communication with beta cells of OB mice. Treatment of beta cells with IL-15, predicted to be released by macrophages, activated signal transducer and activator of transcription (STAT3), which may mediate anti-apoptotic effects. Similar to mice, humans without diabetes possess a greater number of macrophages than those with prediabetes (39 mmol/mol [5.7%] < HbA1c < 46 mmol/mol [6.4%]) and diabetes.
Conclusions/interpretation
Our study indicates that the transcriptional heterogeneity of non-beta cells has an impact on intra-islet crosstalk and participates in beta cell (dys)function.
Data availability
scRNA-seq data from the previous study are available in gene expression omnibus under gene accession number GSE159211 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE159211).
Graphical Abstract
中文翻译:

非 β 胰岛细胞的转录组异质性与小鼠模型中 2 型糖尿病的发生有关
目标/假设
这项工作的目的是了解 2 型糖尿病发病机制早期胰岛中非 β 细胞的作用。
方法
对糖尿病易感性不同的肥胖小鼠品系 (糖尿病耐药 B6.V.Lep ob/ob [OB] 和糖尿病易感新西兰肥胖 [NZO] 小鼠) 的胰岛细胞的单细胞转录组数据采用特异性聚类。
结果
精细的聚类分析揭示了 α 细胞、 delta 细胞和巨噬细胞的几个异质亚群,其中 133 个映射到通过全基因组关联研究确定的人类糖尿病基因。重要的是,在 2 型糖尿病不同阶段供体的人类胰岛数据集中发现了类似的非 β 细胞异质性。NZO 小鼠中占主导地位的 α 细胞簇表现出细胞应激和线粒体能力降低的迹象 (97 个差异表达基因 [DEGs]),而来自这些小鼠的 delta 细胞表现出成熟标志基因 (Hhex 和 Sst) 的表达水平较高,但生长抑素分泌低于 OB 小鼠 (184 个 DEGs)。此外,一簇巨噬细胞在 OB 小鼠胰岛中的丰度几乎是其两倍,并且表现出与 OB 小鼠 β 细胞的广泛细胞间通讯。用 IL-15 处理 β 细胞,预计由巨噬细胞、激活信号转导和转录激活因子 (STAT3) 释放,这可能介导抗细胞凋亡作用。与小鼠类似,没有糖尿病的人比患有糖尿病前期的人(39 mmol/mol [5.7%] < HbA1c < 46 mmol/mol [6.4%])拥有更多的巨噬细胞。
结论/解释
我们的研究表明,非 β 细胞的转录异质性对胰岛内串扰有影响,并参与 β 细胞 (dys) 功能。
数据可用性
先前研究的 scRNA-seq 数据可在基因登录号 GSE159211 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE159211) 下的基因表达综合中获得。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号