当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Origin of the Difference in Proton Transport Direction between Inward and Outward Proton-Pumping Rhodopsins
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.accounts.4c00488 Taito Urui, Yasuhisa Mizutani
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.accounts.4c00488 Taito Urui, Yasuhisa Mizutani
|
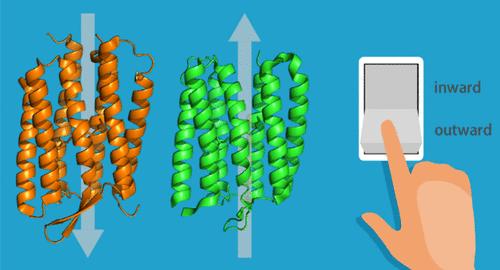
Active transport is a vital and ubiquitous process in biological phenomena. Ion-pumping rhodopsins are light-driven active ion transporters that share a heptahelical transmembrane structural scaffold in which the all-trans retinal chromophore is covalently bonded through a Schiff base to a conserved lysine residue in the seventh transmembrane helix. Bacteriorhodopsin from Halobacterium salinarum was the first ion-pumping rhodopsin to be discovered and was identified as an outward proton-pumping rhodopsin. Since the discovery of bacteriorhodopsin in 1971, many more ion-pumping rhodopsins have been isolated from diverse microorganisms spanning three domains (bacteria, archaea, and eukaryotes) and giant viruses. In addition to proton-pumping rhodopsins, chloride ion- and sodium ion-pumping rhodopsins have also been discovered. Furthermore, diversity of ion-pumping rhodopsins was found in the direction of ion transport; i.e., rhodopsins that pump protons inward have recently been discovered. Very intriguingly, the inward proton-pumping rhodopsins share structural features and many conserved key residues with the outward proton-pumping rhodopsins. However, a central question remains unchanged despite the increasing variety: how and why do the ion-pumping rhodopsins undergo interlocking conformational changes that allow unidirectional ion transfer within proteins? In this regard, it is an effective strategy to compare the structures and their evolutions in the proton-pumping processes of both inward and outward proton-pumping rhodopsins because the comparison sheds light on key elements for the unidirectional proton transport. We elucidated the proton-pumping mechanism of the inward and outward proton-pumping rhodopsins by time-resolved resonance Raman spectroscopy, a powerful technique for tracking the structural evolutions of proteins at work that are otherwise inaccessible.
中文翻译:

向内和向外的质子泵浦视紫红质之间质子传输方向差异的起源
主动运输是生物现象中一个重要且无处不在的过程。离子泵视紫红质是光驱动的活性离子转运蛋白,共享一个七面体跨膜结构支架,其中全反式视网膜发色团通过 Schiff 碱基共价键合到第七个跨膜螺旋中的保守赖氨酸残基上。来自盐酸杆菌的细菌视紫红质是第一个被发现的离子泵视紫红质,并被鉴定为一种向外质子泵送视紫红质。自 1971 年发现细菌视紫红质以来,已经从跨越三个结构域(细菌、古细菌和真核生物)的不同微生物和巨型病毒中分离出更多的离子泵视紫红质。除了质子泵视紫红质外,还发现了氯离子和钠离子泵视紫红质。此外,在离子传输方向上发现了离子泵视紫红质的多样性;即,最近发现了将质子向内泵送的视紫红质。非常有趣的是,向内质子泵送视紫红质与向外质子泵送视紫红质共享结构特征和许多保守的关键残基。然而,尽管种类不断增加,但一个核心问题保持不变:离子泵视紫红质如何以及为什么发生连锁构象变化,从而允许在蛋白质内进行单向离子转移?在这方面,比较向内和向外质子泵送视紫红质的质子泵过程的结构及其演变是一种有效的策略,因为这种比较揭示了单向质子传输的关键要素。 我们通过时间分辨共振拉曼光谱阐明了向内和向外质子泵送视紫红质的质子泵机制,这是一种强大的技术,用于跟踪工作中蛋白质的结构演变,否则无法获得。
更新日期:2024-11-07
中文翻译:

向内和向外的质子泵浦视紫红质之间质子传输方向差异的起源
主动运输是生物现象中一个重要且无处不在的过程。离子泵视紫红质是光驱动的活性离子转运蛋白,共享一个七面体跨膜结构支架,其中全反式视网膜发色团通过 Schiff 碱基共价键合到第七个跨膜螺旋中的保守赖氨酸残基上。来自盐酸杆菌的细菌视紫红质是第一个被发现的离子泵视紫红质,并被鉴定为一种向外质子泵送视紫红质。自 1971 年发现细菌视紫红质以来,已经从跨越三个结构域(细菌、古细菌和真核生物)的不同微生物和巨型病毒中分离出更多的离子泵视紫红质。除了质子泵视紫红质外,还发现了氯离子和钠离子泵视紫红质。此外,在离子传输方向上发现了离子泵视紫红质的多样性;即,最近发现了将质子向内泵送的视紫红质。非常有趣的是,向内质子泵送视紫红质与向外质子泵送视紫红质共享结构特征和许多保守的关键残基。然而,尽管种类不断增加,但一个核心问题保持不变:离子泵视紫红质如何以及为什么发生连锁构象变化,从而允许在蛋白质内进行单向离子转移?在这方面,比较向内和向外质子泵送视紫红质的质子泵过程的结构及其演变是一种有效的策略,因为这种比较揭示了单向质子传输的关键要素。 我们通过时间分辨共振拉曼光谱阐明了向内和向外质子泵送视紫红质的质子泵机制,这是一种强大的技术,用于跟踪工作中蛋白质的结构演变,否则无法获得。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号