当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Optimized Synthesis of an Abemaciclib Intermediate: Improved Conditions for a Miyaura Borylation/Suzuki Coupling Process
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.oprd.4c00381 Michael P. Carroll, Aobha Hickey, Ava Rogers, Cáoimhe J. Niland, Rachel A. O’Sullivan, Nachimuthu Muniraj, Kevin F. O’Sullivan, Patrick J. Guiry, Michael M. Murray
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2024-11-07 , DOI: 10.1021/acs.oprd.4c00381 Michael P. Carroll, Aobha Hickey, Ava Rogers, Cáoimhe J. Niland, Rachel A. O’Sullivan, Nachimuthu Muniraj, Kevin F. O’Sullivan, Patrick J. Guiry, Michael M. Murray
|
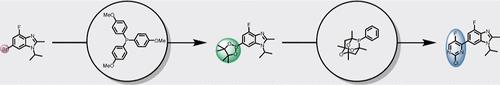
Improved reaction conditions have been developed for a telescoped Miyaura borylation/Suzuki coupling process, which is utilized in the synthesis of an abemaciclib intermediate. Key improvements include the in situ generation of a lipophilic base and tailored ligand selection for each palladium-catalyzed step. Optimizing ligand choice significantly reduced aryl scrambling, a major source of impurities in the borylation step. Additionally, the process improvements led to shortened reaction times and lower palladium loadings, resulting in a more efficient, higher-yielding process.
中文翻译:

Abemaciclib 中间体的优化合成:改善 Miyaura 硼酸化/Suzuki 偶联工艺的条件
已经为望远镜式 Miyaura 硼酸化/Suzuki 偶联工艺开发了改进的反应条件,该工艺用于合成 abemaciclib 中间体。主要改进包括原位生成亲脂性碱基和为每个钯催化步骤量身定制的配体选择。优化配体选择可显著减少芳基加扰,这是硼酸化步骤中杂质的主要来源。此外,工艺改进缩短了反应时间并降低了钯含量,从而实现了更高效、更高产量的工艺。
更新日期:2024-11-07
中文翻译:

Abemaciclib 中间体的优化合成:改善 Miyaura 硼酸化/Suzuki 偶联工艺的条件
已经为望远镜式 Miyaura 硼酸化/Suzuki 偶联工艺开发了改进的反应条件,该工艺用于合成 abemaciclib 中间体。主要改进包括原位生成亲脂性碱基和为每个钯催化步骤量身定制的配体选择。优化配体选择可显著减少芳基加扰,这是硼酸化步骤中杂质的主要来源。此外,工艺改进缩短了反应时间并降低了钯含量,从而实现了更高效、更高产量的工艺。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号