当前位置:
X-MOL 学术
›
J. Chem. Inf. Model.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Exploring the Impact of Physiological C-Terminal Truncation on α-Synuclein Conformations to Unveil Mechanisms Regulating Pathological Aggregation
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-11-06 , DOI: 10.1021/acs.jcim.4c01839 Fengjuan Huang, Jiajia Yan, Huan Xu, Ying Wang, Xiaohan Zhang, Yu Zou, Jiangfang Lian, Feng Ding, Yunxiang Sun
Journal of Chemical Information and Modeling ( IF 5.6 ) Pub Date : 2024-11-06 , DOI: 10.1021/acs.jcim.4c01839 Fengjuan Huang, Jiajia Yan, Huan Xu, Ying Wang, Xiaohan Zhang, Yu Zou, Jiangfang Lian, Feng Ding, Yunxiang Sun
|
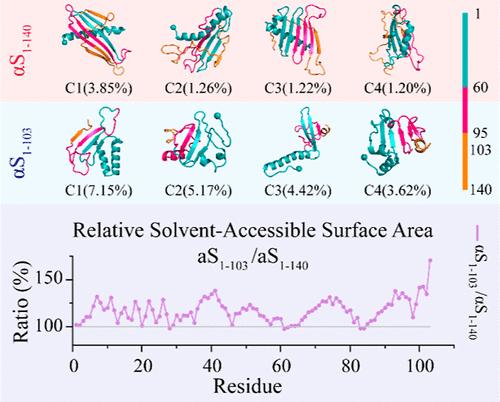
Emerging evidence suggests that physiological C-terminal truncation of α-synuclein (αS) plays a critical role in regulating liquid–liquid phase separation and promoting amyloid aggregation, processes implicated in neurodegenerative diseases such as Parkinson’s disease (PD). However, the molecular mechanisms through which C-terminal truncation influences αS conformation and modulates its aggregation remain poorly understood. In this study, we investigated the impact of C-terminal truncation on αS conformational dynamics by comparing full-length αS1–140 with truncated αS1–103 monomers using atomistic discrete molecular dynamics simulations. Our findings revealed that both αS1–140 and αS1–103 primarily adopted helical conformations around residues 7–32, while residues 36–95, located in the second half of the N-terminal and NAC domains, predominantly formed a dynamic β-sheet core. The C-terminus of αS1–140 was largely unstructured and dynamically wrapped around the β-sheet core. While residues 1–95 exhibited similar secondary structure propensities in both αS1–140 and αS1–103, the dynamic capping by the C-terminus in αS1–140 slightly enhanced β-sheet formation around residues 36–95. In contrast, key aggregation-driving regions (residues 2–9, 36–42, 45–57, and 68–78) were dynamically shielded by the C-terminus in αS1–140, reducing their exposure and potentially preventing interpeptide interactions that drive aggregation. C-terminal truncation, on the other hand, increased the exposed surface area of these aggregation-prone regions, thereby enhancing interpeptide interactions, phase separation, and amyloid aggregation. Overall, our simulations provide valuable insights into the conformational effects of C-terminal truncation on αS and its role in promoting pathological aggregation.
中文翻译:

探索生理性 C 末端截短对 α-突触核蛋白构象的影响,以揭示调节病理聚集的机制
新出现的证据表明,α-突触核蛋白 (αS) 的生理性 C 末端截短在调节液-液相分离和促进淀粉样蛋白聚集方面起关键作用,这些过程与帕金森病 (PD) 等神经退行性疾病有关。然而,C 端截短影响 αS 构象并调节其聚集的分子机制仍然知之甚少。在这项研究中,我们通过使用原子离散分子动力学模拟比较全长 αS1-140 与截短的 αS1-103 单体,研究了 C 末端截断对 αS 构象动力学的影响。我们的研究结果表明,αS1-140 和 αS1-103 主要采用围绕残基 7-32 的螺旋构象,而位于 N 末端和 NAC 结构域后半部分的残基 36-95 主要形成动态 β 片核心。αS1-140 的 C 端在很大程度上是非结构化的,并动态包裹在 β 片核周围。虽然残基 1-95 在 αS1-140 和 αS1-103 中表现出相似的二级结构倾向,但 αS1-140 中 C 端的动态加帽略微增强了残基 36-95 周围的β片形成。相比之下,关键的聚集驱动区域(残基 2-9、36-42、45-57 和 68-78)被 αS1-140 中的 C 端动态屏蔽,减少了它们的暴露,并可能阻止了驱动聚集的肽间相互作用。另一方面,C 端截断增加了这些易聚集区域的暴露表面积,从而增强了肽间相互作用、相分离和淀粉样蛋白聚集。 总体而言,我们的模拟为 C 端截断对 αS 的构象效应及其在促进病理聚集中的作用提供了有价值的见解。
更新日期:2024-11-07
中文翻译:

探索生理性 C 末端截短对 α-突触核蛋白构象的影响,以揭示调节病理聚集的机制
新出现的证据表明,α-突触核蛋白 (αS) 的生理性 C 末端截短在调节液-液相分离和促进淀粉样蛋白聚集方面起关键作用,这些过程与帕金森病 (PD) 等神经退行性疾病有关。然而,C 端截短影响 αS 构象并调节其聚集的分子机制仍然知之甚少。在这项研究中,我们通过使用原子离散分子动力学模拟比较全长 αS1-140 与截短的 αS1-103 单体,研究了 C 末端截断对 αS 构象动力学的影响。我们的研究结果表明,αS1-140 和 αS1-103 主要采用围绕残基 7-32 的螺旋构象,而位于 N 末端和 NAC 结构域后半部分的残基 36-95 主要形成动态 β 片核心。αS1-140 的 C 端在很大程度上是非结构化的,并动态包裹在 β 片核周围。虽然残基 1-95 在 αS1-140 和 αS1-103 中表现出相似的二级结构倾向,但 αS1-140 中 C 端的动态加帽略微增强了残基 36-95 周围的β片形成。相比之下,关键的聚集驱动区域(残基 2-9、36-42、45-57 和 68-78)被 αS1-140 中的 C 端动态屏蔽,减少了它们的暴露,并可能阻止了驱动聚集的肽间相互作用。另一方面,C 端截断增加了这些易聚集区域的暴露表面积,从而增强了肽间相互作用、相分离和淀粉样蛋白聚集。 总体而言,我们的模拟为 C 端截断对 αS 的构象效应及其在促进病理聚集中的作用提供了有价值的见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号