当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiocyanoalkylation of alkenes via dual photoredox and copper catalysis
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-07 , DOI: 10.1039/d4qo01864h Xu Wang, Bi-Yin Xiao, Qi-Xuan Jiang, Wei Huang, Feng-Hua Zhang
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2024-11-07 , DOI: 10.1039/d4qo01864h Xu Wang, Bi-Yin Xiao, Qi-Xuan Jiang, Wei Huang, Feng-Hua Zhang
|
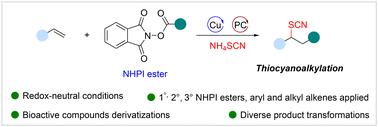
Organic thiocyanates are commonly used as essential organic synthesis intermediates and are widely present in various drug molecules and bioactive molecules. Herein, a copper- and photoredox-catalyzed thiocyanoalkylation reaction of terminal alkenes with NHPI esters and NH4SCN to access organic thiocyanates is described. This protocol employs the more readily accessible NHPI ester as a radical precursor under redox-neutral conditions. Moreover, the reaction exhibits high compatibility with diverse primary, secondary and tertiary NHPI esters. The late-stage modification of some drugs and structurally complex natural products enables the potential application in organic synthesis.
中文翻译:

烯烃的硫氰基烷基化反应通过双重光氧化还原和铜催化
有机硫氰酸盐常用作必需的有机合成中间体,广泛存在于各种药物分子和生物活性分子中。在此,描述了末端烯烃与 NHPI 酯和 NH4SCN 的铜和光氧化还原催化的硫氰烷基化反应以获得有机硫氰酸盐。该方案在氧化还原中性条件下使用更容易获得的 NHPI 酯作为自由基前体。此外,该反应与多种伯、仲和叔 NHPI 酯表现出高度相容性。一些药物和结构复杂的天然产物的后期修饰使其在有机合成中具有潜在的应用。
更新日期:2024-11-07
中文翻译:

烯烃的硫氰基烷基化反应通过双重光氧化还原和铜催化
有机硫氰酸盐常用作必需的有机合成中间体,广泛存在于各种药物分子和生物活性分子中。在此,描述了末端烯烃与 NHPI 酯和 NH4SCN 的铜和光氧化还原催化的硫氰烷基化反应以获得有机硫氰酸盐。该方案在氧化还原中性条件下使用更容易获得的 NHPI 酯作为自由基前体。此外,该反应与多种伯、仲和叔 NHPI 酯表现出高度相容性。一些药物和结构复杂的天然产物的后期修饰使其在有机合成中具有潜在的应用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号