当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synergistic coupling of a CuNi alloy with a CoFe LDH heterostructure on nickel foam toward high-efficiency overall water splitting
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-11-07 , DOI: 10.1039/d4ta05681g Dan Wang, Yuan Chu, Youzheng Wu, Mengkang Zhu, Lin Pan, Ruopeng Li, Yukai Chen, Wenchang Wang, Naotoshi Mitsuzaki, Zhidong Chen
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-11-07 , DOI: 10.1039/d4ta05681g Dan Wang, Yuan Chu, Youzheng Wu, Mengkang Zhu, Lin Pan, Ruopeng Li, Yukai Chen, Wenchang Wang, Naotoshi Mitsuzaki, Zhidong Chen
|
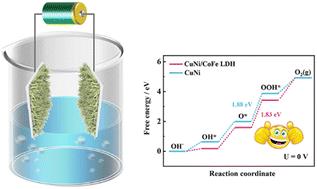
Accelerating the kinetics of the oxygen evolution reaction (OER) and hydrogen evolution reaction (HER) is vital for high-efficiency green hydrogen production. However, developing cost-effective and highly active bifunctional catalysts for overall water splitting electrolysis remains a huge challenge. Herein, the CuNi/CoFe LDH heterostructure is synthesized in situ on nickel foam (CuNi/CoFe LDH@NF) by a simple two-step electrodeposition process. The synergy of the CuNi alloy and CoFe LDH optimizes the electron distribution at the interface and improves the intrinsic activity of the HER/OER. Consequently, the optimal CuNi/CoFe LDH@NF bifunctional catalyst displays low overpotentials of 56 mV (10 mA cm−2) and 268 mV (50 mA cm−2) for the HER and OER, respectively, along with high stability in alkaline electrolyte. Remarkably, CuNi/CoFe LDH@NF as the cathode and anode requires a low voltage (1.49 V) to achieve 10 mA cm−2 for overall water splitting. Meanwhile, it also displays favorable stability for operation for 17 h (50 mA cm−2) without obvious decline of the cell voltage. Density functional theory calculations indicate that constructing heterojunction interfaces promotes the redistribution of interface electrons and optimizes the free energy of adsorbed intermediates, thereby reducing the energy barrier of the rate-determining step (from *O to *OOH).
中文翻译:

CuNi 合金与 CoFe LDH 异质结构在泡沫镍上的协同耦合,以实现高效的整体分解水
加速析氧反应 (OER) 和析氢反应 (HER) 的动力学对于高效绿色氢气生产至关重要。然而,开发经济高效且高活性的双功能催化剂用于整体分解水电解仍然是一项巨大的挑战。在此,CuNi/CoFe LDH 异质结构是通过简单的两步电沉积工艺在泡沫镍 (CuNi/CoFe LDH@NF) 上原位合成的。CuNi 合金和 CoFe LDH 的协同作用优化了界面处的电子分布,并提高了 HER/OER 的本征活性。因此,最佳的 CuNi/CoFe LDH@NF双功能催化剂对 HER 和 OER 分别显示出 56 mV (10 mA cm-2) 和 268 mV (50 mA cm-2) 的低过电位,以及在碱性电解质中的高稳定性。值得注意的是,作为阴极和阳极的 CuNi/CoFe LDH@NF需要低电压 (1.49 V) 才能达到 10 mA cm-2 的整体水分解。同时,它还显示出良好的运行稳定性 17 小时 (50 mA cm-2),电池电压没有明显下降。密度泛函理论计算表明,构建异质结界面促进了界面电子的重新分布并优化了吸附中间体的自由能,从而降低了速率决定步骤的能量势垒(从 *O 到 *OOH)。
更新日期:2024-11-07
中文翻译:

CuNi 合金与 CoFe LDH 异质结构在泡沫镍上的协同耦合,以实现高效的整体分解水
加速析氧反应 (OER) 和析氢反应 (HER) 的动力学对于高效绿色氢气生产至关重要。然而,开发经济高效且高活性的双功能催化剂用于整体分解水电解仍然是一项巨大的挑战。在此,CuNi/CoFe LDH 异质结构是通过简单的两步电沉积工艺在泡沫镍 (CuNi/CoFe LDH@NF) 上原位合成的。CuNi 合金和 CoFe LDH 的协同作用优化了界面处的电子分布,并提高了 HER/OER 的本征活性。因此,最佳的 CuNi/CoFe LDH@NF双功能催化剂对 HER 和 OER 分别显示出 56 mV (10 mA cm-2) 和 268 mV (50 mA cm-2) 的低过电位,以及在碱性电解质中的高稳定性。值得注意的是,作为阴极和阳极的 CuNi/CoFe LDH@NF需要低电压 (1.49 V) 才能达到 10 mA cm-2 的整体水分解。同时,它还显示出良好的运行稳定性 17 小时 (50 mA cm-2),电池电压没有明显下降。密度泛函理论计算表明,构建异质结界面促进了界面电子的重新分布并优化了吸附中间体的自由能,从而降低了速率决定步骤的能量势垒(从 *O 到 *OOH)。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号