当前位置:
X-MOL 学术
›
Dalton Trans.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis of organo-uranium(II) species in the gas-phase using reactions between [UH]+ and nitriles
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-07 , DOI: 10.1039/d4dt02508c Justin G. Terhorst, Theodore A. Corcovilos, Samuel J. Lenze, Michael J. van Stipdonk
Dalton Transactions ( IF 3.5 ) Pub Date : 2024-11-07 , DOI: 10.1039/d4dt02508c Justin G. Terhorst, Theodore A. Corcovilos, Samuel J. Lenze, Michael J. van Stipdonk
|
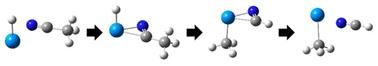
One challenge in the quest to map the intrinsic reactivity of model actinide species has been the controlled synthesis of organo-actinide ions in the gas phase. We report here evidence that a series of gas-phase, σ-bonded [U–R]+ species (where R = CH3, C2H3, C2H5, C3H7, or C5H6) can be generated for subsequent study of ion-molecule chemistry by using preparative tandem mass spectrometry (PTMSn) via ion-molecule reactions between [UH]+ and a series of nitriles. Density functional theory calculations support the hypothesis that the [U–R]+ ions are created in a pathway that involves intramolecular hydride attack and the elimination of neutral HCN. Subsequent reactivity experiments revealed that the [UCH3]+ readily undergoes hydrolysis, yielding cationic uranium hydroxide ([UOH]+) and methane (CH4). Other possible reaction pathways, such as the spontaneous rearrangement to [HU![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2]+, are shown by theoretical calculations to have energy barriers, strengthening the evidence for the formation of a σ-bonded [U–CH3]+ complex in the gas-phase.
CH2]+, are shown by theoretical calculations to have energy barriers, strengthening the evidence for the formation of a σ-bonded [U–CH3]+ complex in the gas-phase.
中文翻译:

利用 [UH]+ 和腈之间的反应在气相中合成有机铀 (II) 物质
在寻求绘制模型锕系元素物种的内禀反应性的过程中,一个挑战是在气相中控制合成有机锕系元素离子。我们在这里报告的证据表明,通过使用制备串联质谱 (PTMSn),可以产生一系列气相、σ键 [U–R]+ 物质(其中 R = CH3、C2H3、C2H5、C3H7 或 C5H6),用于离子分子化学的后续研究[UH]+ 与一系列腈之间的离子-分子反应。密度泛函理论计算支持以下假设:[U–R]+ 离子是在涉及分子内氢化物攻击和中性 HCN 消除的途径中产生的。随后的反应性实验表明,[UCH3]+ 容易发生水解,产生阳离子氢氧化铀 ([UOH]+) 和甲烷 (CH4)。理论计算表明,其他可能的反应途径,例如自发重排为 [胡![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2]+,具有能垒,这加强了在气相中形成σ键 [U–CH3]+ 络合物的证据。
CH2]+,具有能垒,这加强了在气相中形成σ键 [U–CH3]+ 络合物的证据。
更新日期:2024-11-12
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH2]+, are shown by theoretical calculations to have energy barriers, strengthening the evidence for the formation of a σ-bonded [U–CH3]+ complex in the gas-phase.
CH2]+, are shown by theoretical calculations to have energy barriers, strengthening the evidence for the formation of a σ-bonded [U–CH3]+ complex in the gas-phase.
中文翻译:

利用 [UH]+ 和腈之间的反应在气相中合成有机铀 (II) 物质
在寻求绘制模型锕系元素物种的内禀反应性的过程中,一个挑战是在气相中控制合成有机锕系元素离子。我们在这里报告的证据表明,通过使用制备串联质谱 (PTMSn),可以产生一系列气相、σ键 [U–R]+ 物质(其中 R = CH3、C2H3、C2H5、C3H7 或 C5H6),用于离子分子化学的后续研究[UH]+ 与一系列腈之间的离子-分子反应。密度泛函理论计算支持以下假设:[U–R]+ 离子是在涉及分子内氢化物攻击和中性 HCN 消除的途径中产生的。随后的反应性实验表明,[UCH3]+ 容易发生水解,产生阳离子氢氧化铀 ([UOH]+) 和甲烷 (CH4)。理论计算表明,其他可能的反应途径,例如自发重排为 [胡

































 京公网安备 11010802027423号
京公网安备 11010802027423号