当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of the Coreactant on the Dual-Source Behavior of Lithium Hexamethyldisilazide for ALD Li-Containing Films
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-11-06 , DOI: 10.1021/acs.jpcc.4c05987 M. J. Pieters, L. Bartel, C. van Helvoirt, M. Creatore
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2024-11-06 , DOI: 10.1021/acs.jpcc.4c05987 M. J. Pieters, L. Bartel, C. van Helvoirt, M. Creatore
|
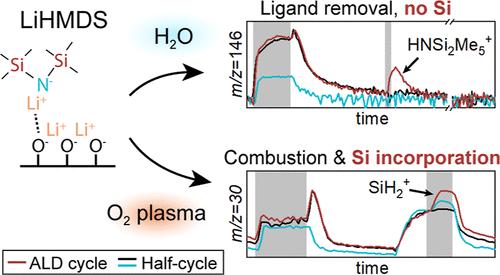
Atomic layer deposition (ALD) of Li-containing thin films is deemed as highly relevant for the development of next-generation Li-ion batteries. Lithium hexamethyldisilazide (LiHMDS), as Li-containing precursor, is preferred over the widely used lithium tert-butoxide because of its lower melting point of 70 °C. However, the presence of silyl groups in the LiHMDS chemical structure can result in the undesired incorporation of Si in the film. Therefore, understanding the reaction mechanism of LiHMDS is required to control its dual-source behavior and grow Si-free Li-containing thin films. For this purpose LiHMDS was combined with O2 plasma or water as coreactant. In situ spectroscopic ellipsometry (SE) and X-ray photoelectron spectroscopy (XPS) revealed that using O2 plasma as coreactant results in linear growth and Si-containing films, whereas using H2O as coreactant leads to fast, nonsurface-reaction-limited growth and Si-free films. To shed light on the role of the coreactant on the reaction mechanism of LiHMDS, in situ studies by time-resolved quadrupole mass spectrometry (QMS) were performed on the O2 plasma and H2O-based ALD processes. Measurements taken during full ALD cycles and half-cycles were carefully compared to identify which half-cycle surface reaction products lead to silicon incorporation in the film. The QMS results of the LiHMDS + H2O process showed that LiHMDS both chemisorbs and physisorbs. Furthermore, it is concluded that Si incorporation occurs during the O2 plasma step, when the physisorbed ligands are combusted and Si-containing products are redeposited. This work also demonstrates that the incorporated Si can be abstracted from the film by means of a H2 plasma step following the O2 plasma step. These insights on the role of the coreactant in the synthesis of Li-containing films contribute to the development of LiHMDS-based ALD processes for Li-ion battery applications.
中文翻译:

共反应物对六甲基二硅氮化锂对 ALD 含锂薄膜双源行为的影响
含锂薄膜的原子层沉积 (ALD) 被认为与下一代锂离子电池的开发高度相关。六甲基二硅氮化锂 (LiHMDS) 作为含锂前驱体,由于其熔点较低,为 70 °C,优于广泛使用的叔丁醇锂。 然而,LiHMDS 化学结构中存在甲硅烷基会导致 Si 意外掺入薄膜中。因此,需要了解 LiHMDS 的反应机理来控制其双源行为并生长无硅的含锂薄膜。为此,LiHMDS 与 O2 等离子体或水作为共反应物结合。原位椭圆偏振光谱 (SE) 和 X 射线光电子能谱 (XPS) 表明,使用 O2 等离子体作为共反应物会产生线性生长和含硅薄膜,而使用 H2O 作为共反应物会导致快速、非表面反应限制生长和无硅薄膜。为了阐明共反应物对 LiHMDS 反应机理的作用,通过时间分辨四极杆质谱 (QMS) 对 O2 等离子体和 H2O 基原子层沉积工艺进行了原位研究。仔细比较了在整个 ALD 循环和半循环期间进行的测量,以确定哪些半循环表面反应产物导致硅掺入薄膜中。LiHMDS + H2O 工艺的 QMS 结果表明,LiHMDS 既是化学吸附又是物理吸附。此外,得出结论,Si 掺入发生在 O2 等离子体步骤中,此时物理吸附配体被燃烧并且含 Si 的产物被重新沉积。 这项工作还表明,在 O2 等离子体步骤之后,可以通过 H2 等离子体步骤从薄膜中提取掺入的 Si。这些关于共反应物在含锂薄膜合成中的作用的见解有助于开发用于锂离子电池应用的基于 LiHMDS 的 ALD 工艺。
更新日期:2024-11-06
中文翻译:

共反应物对六甲基二硅氮化锂对 ALD 含锂薄膜双源行为的影响
含锂薄膜的原子层沉积 (ALD) 被认为与下一代锂离子电池的开发高度相关。六甲基二硅氮化锂 (LiHMDS) 作为含锂前驱体,由于其熔点较低,为 70 °C,优于广泛使用的叔丁醇锂。 然而,LiHMDS 化学结构中存在甲硅烷基会导致 Si 意外掺入薄膜中。因此,需要了解 LiHMDS 的反应机理来控制其双源行为并生长无硅的含锂薄膜。为此,LiHMDS 与 O2 等离子体或水作为共反应物结合。原位椭圆偏振光谱 (SE) 和 X 射线光电子能谱 (XPS) 表明,使用 O2 等离子体作为共反应物会产生线性生长和含硅薄膜,而使用 H2O 作为共反应物会导致快速、非表面反应限制生长和无硅薄膜。为了阐明共反应物对 LiHMDS 反应机理的作用,通过时间分辨四极杆质谱 (QMS) 对 O2 等离子体和 H2O 基原子层沉积工艺进行了原位研究。仔细比较了在整个 ALD 循环和半循环期间进行的测量,以确定哪些半循环表面反应产物导致硅掺入薄膜中。LiHMDS + H2O 工艺的 QMS 结果表明,LiHMDS 既是化学吸附又是物理吸附。此外,得出结论,Si 掺入发生在 O2 等离子体步骤中,此时物理吸附配体被燃烧并且含 Si 的产物被重新沉积。 这项工作还表明,在 O2 等离子体步骤之后,可以通过 H2 等离子体步骤从薄膜中提取掺入的 Si。这些关于共反应物在含锂薄膜合成中的作用的见解有助于开发用于锂离子电池应用的基于 LiHMDS 的 ALD 工艺。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号