当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Discovery of novel fused-heterocycle-bearing diarypyrimidine derivatives as HIV-1 potent NNRTIs targeting tolerant region I for enhanced antiviral activity and resistance profile
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-07 , DOI: 10.1016/j.ejmech.2024.117033 Jiaojiao Dai, Xiangyi Jiang, Heng Gao, Boshi Huang, Erik De Clercq, Christophe Pannecouque, Shaoqing Du, Xinyong Liu, Peng Zhan
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-11-07 , DOI: 10.1016/j.ejmech.2024.117033 Jiaojiao Dai, Xiangyi Jiang, Heng Gao, Boshi Huang, Erik De Clercq, Christophe Pannecouque, Shaoqing Du, Xinyong Liu, Peng Zhan
|
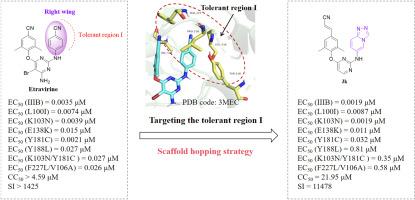
As an important part of anti-AIDS therapy, HIV-1 non-nucleoside reverse transcriptase inhibitors are plagued by resistance and toxicity issues. Taking our reported XJ-18b1 as lead compound, we designed a series of novel diarypyrimidine derivatives by employing a scaffold hopping strategy to discover potent NNRTIs with improved anti-resistance properties and drug-like profiles. The most active compound 3k exhibited prominent inhibitory activity against wild-type HIV-1 (EC50 = 0.0019 μM) and common mutant strains including K103 N (EC50 = 0.0019 μM), L100I (EC50 = 0.0087 μM), E138K (EC50 = 0.011 μM), along with low cytotoxicity and high selectivity index (CC50 = 21.95 μM, SI = 11478). Additionally, compound 3k demonstrated antiviral activity against HIV-2 with EC50 value of 6.14 μM. The enzyme-linked immunosorbent assay validated that 3k could significantly inhibit the activity of HIV-1 reverse transcriptase (IC50 = 0.025 μM). Furthermore, molecular dynamics simulation studies were performed to illustrate the potential binding mode and binding free energy of the RT-3k complex, and in silico prediction revealed that 3k possessed favorable drug-like profiles. Collectively, 3k proved to be a promising lead compound for further optimization to obtain anti-HIV drug candidates.
中文翻译:

发现新型含熔杂环二芳嘧啶衍生物作为靶向耐受区域 I 的 HIV-1 强效 NNRTIs,以增强抗病毒活性和耐药性
作为抗 AIDS 治疗的重要组成部分,HIV-1 非核苷逆转录酶抑制剂受到耐药性和毒性问题的困扰。以我们报道的 XJ-18b1 为先导化合物,我们通过采用支架跳跃策略设计了一系列新型二芳基嘧啶衍生物,以发现具有改进的抗耐药特性和药物样特征的有效 NNRTI。最活跃的化合物 3k 对野生型 HIV-1 (EC50 = 0.0019 μM) 和常见突变菌株(包括 K103 N (EC50 = 0.0019 μM)、L100I (EC50 = 0.0087 μM)、E138K (EC50 = 0.011 μM) 表现出显著的抑制活性,以及低细胞毒性和高选择性指数 (CC50 = 21.95 μM,SI = 11478)。此外,化合物 3k 对 HIV-2 具有抗病毒活性,EC50 值为 6.14 μM。酶联免疫吸附试验证实 3k 可显著抑制 HIV-1 逆转录酶活性 (IC50 = 0.025 μM)。此外,进行了分子动力学模拟研究以说明 RT-3k 复合物的潜在结合模式和结合自由能,计算机预测显示 3k 具有良好的药物样特征。总的来说,3k 被证明是一种很有前途的先导化合物,可用于进一步优化以获得抗 HIV 候选药物。
更新日期:2024-11-07
中文翻译:

发现新型含熔杂环二芳嘧啶衍生物作为靶向耐受区域 I 的 HIV-1 强效 NNRTIs,以增强抗病毒活性和耐药性
作为抗 AIDS 治疗的重要组成部分,HIV-1 非核苷逆转录酶抑制剂受到耐药性和毒性问题的困扰。以我们报道的 XJ-18b1 为先导化合物,我们通过采用支架跳跃策略设计了一系列新型二芳基嘧啶衍生物,以发现具有改进的抗耐药特性和药物样特征的有效 NNRTI。最活跃的化合物 3k 对野生型 HIV-1 (EC50 = 0.0019 μM) 和常见突变菌株(包括 K103 N (EC50 = 0.0019 μM)、L100I (EC50 = 0.0087 μM)、E138K (EC50 = 0.011 μM) 表现出显著的抑制活性,以及低细胞毒性和高选择性指数 (CC50 = 21.95 μM,SI = 11478)。此外,化合物 3k 对 HIV-2 具有抗病毒活性,EC50 值为 6.14 μM。酶联免疫吸附试验证实 3k 可显著抑制 HIV-1 逆转录酶活性 (IC50 = 0.025 μM)。此外,进行了分子动力学模拟研究以说明 RT-3k 复合物的潜在结合模式和结合自由能,计算机预测显示 3k 具有良好的药物样特征。总的来说,3k 被证明是一种很有前途的先导化合物,可用于进一步优化以获得抗 HIV 候选药物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号