Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2024-11-06 , DOI: 10.1002/adsc.202401175 Ismat Nawaz, Maryam Nawaz, Rahman Shah Zaib Saleem, Ghayoor Abbas Chotana
|
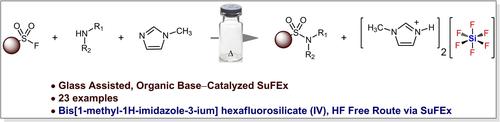
An environmentally benign route for the synthesis of sulfonamides via Sulfur (VI) Fluoride Exchange (SuFEx) chemistry utilizing N-methylimidazole, that simultaneously act as a base, precatalyst, HF by-product scavenger, as well as solvent, is described. This one-step sulfonamide synthesis exhibits excellent reactivity toward highly electron-deficient and less nucleophilic anilines as well as aminopyridines while tolerating a wide range of functional groups. In addition to the desired sulfonamide target, we also isolated an ionic salt as the sole side product from the reaction mixture that has been identified as bis[1-methyl-1H-imidazole-3-ium] hexafluorosilicate (IV). The glass surface of reaction vessel is acting as the source of silicon present in the isolated side product. Formation of hexafluorosilicate salt is also facilitating the consumption of sulfonyl fluoride for sulfonamide synthesis. Optimization of the reaction under various conditions, as well as the isolation of the bis[1-methyl-1H-imidazole-3-ium] hexafluorosilicate (IV) salt, highlight the crucial role of N-methylimidazole and support the glass-assisted approach. Besides the first example of glass–assisted SuFEx catalyzed by benign organic bases, this reaction also offers an alternative route for accessing protic hexafluorosilicate-based molten salts without employing external HF. The synthetic utility of this SuFEx route for late-stage functionalization is also demonstrated.
中文翻译:

通过玻璃辅助有机催化进行硫 (VI) 氟化物交换 (SuFEx)
描述了一种利用 N-甲基咪唑通过硫 (VI) 氟化物交换 (SuFEx) 化学合成磺胺类药物的环保路线,该化学反应同时用作碱、预催化剂、HF 副产物清除剂以及溶剂。这种一步法磺酰胺合成对高度缺电子和亲核性较低的苯胺以及氨基吡啶表现出优异的反应性,同时耐受广泛的官能团。除了所需的磺胺靶标外,我们还从反应混合物中分离出一种离子盐作为唯一的副产物,该离子盐已被鉴定为双[1-甲基-1H-咪唑-3-铉] 六氟硅酸盐 (IV)。反应容器的玻璃表面充当隔离副产物中存在的硅源。六氟硅酸盐的形成也促进了磺酰氟在磺酰胺合成中的消耗。各种条件下的反应优化,以及双[1-甲基-1H-咪唑-3-铉]六氟硅酸盐 (IV) 盐的分离,突出了 N-甲基咪唑的关键作用,并支持玻璃辅助方法。除了由良性有机碱催化的玻璃辅助 SuFEx 的第一个例子外,该反应还提供了一种无需使用外部 HF 即可获得质子六氟硅酸盐基熔盐的替代途径。还证明了这种 SuFEx 路线对后期官能团化的合成效用。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号