当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Single Amine or Guanidine Modification on Norvancomycin and Vancomycin to Overcome Multidrug-Resistance through Augmented Lipid II Binding and Increased Membrane Activity
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-11-06 , DOI: 10.1021/acs.jmedchem.4c02196 Xiaolei Bian, Zhifu Chen, Fang Li, Yuanyuan Xie, Yi Li, Youhong Luo, Xiangman Zou, Hui Wang, Jingwen Zhang, Xiaowen Wang, Jinyong Zhang, Dongliang Guan
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-11-06 , DOI: 10.1021/acs.jmedchem.4c02196 Xiaolei Bian, Zhifu Chen, Fang Li, Yuanyuan Xie, Yi Li, Youhong Luo, Xiangman Zou, Hui Wang, Jingwen Zhang, Xiaowen Wang, Jinyong Zhang, Dongliang Guan
|
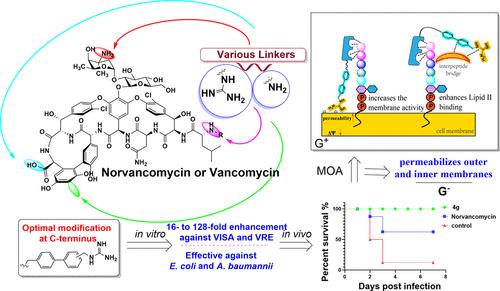
Vancomycin and norvancomycin have diminished antibacterial efficacy due to acquired or intrinsic resistance from mutations in the terminal dipeptide of lipid II in Gram-positive bacteria or failure to penetrate into the periplasm in Gram-negative bacteria. Herein, we rationally designed and synthesized a series of vancomycin analogues bearing single amine or guanidine functionality, altering various linkers and modification sites, to combat the resistance. Extensive antibacterial screening was performed to delineate a comprehensive SAR. Many derivatives revitalized the activity in vitro, exhibiting a 4–128-fold or 2–16-fold enhancement against the acquired or intrinsic resistance with lower toxicity. Significantly, the optimal compound 4g demonstrated greater pharmacokinetic and pharmacodynamic profiles. Further studies uncovered additional independent and synergistic mechanisms for 4g, including the enhanced membrane activity and augmented inhibition of peptidoglycan biosynthesis via increased lipid II binding, highlighting its potential as a future lead candidate to replenish the glycopeptide antibiotic arsenal.
中文翻译:

去甲古霉素和万古霉素上的单胺或胍修饰通过增强脂质 II 结合和增加膜活性来克服多重耐药性
万古霉素和去甲古霉素的抗菌功效降低,这是由于革兰氏阳性菌脂质 II 末端二肽突变或革兰氏阴性菌未能渗透到周质引起的获得性或内在耐药性。在此,我们合理设计合成了一系列具有单胺或胍官能团的万古霉素类似物,改变了各种连接子和修饰位点,以对抗耐药性。进行了广泛的抗菌筛查以描绘全面的 SAR。许多衍生物在体外恢复了活性,对获得性或内在耐药性表现出 4-128 倍或 2-16 倍的增强,毒性较低。值得注意的是,最佳化合物 4g 表现出更好的药代动力学和药效学特征。进一步的研究揭示了 4g 的其他独立和协同机制,包括通过增加脂质 II 结合增强膜活性和增强对肽聚糖生物合成的抑制,突出了其作为未来补充糖肽抗生素库的主要候选者的潜力。
更新日期:2024-11-07
中文翻译:

去甲古霉素和万古霉素上的单胺或胍修饰通过增强脂质 II 结合和增加膜活性来克服多重耐药性
万古霉素和去甲古霉素的抗菌功效降低,这是由于革兰氏阳性菌脂质 II 末端二肽突变或革兰氏阴性菌未能渗透到周质引起的获得性或内在耐药性。在此,我们合理设计合成了一系列具有单胺或胍官能团的万古霉素类似物,改变了各种连接子和修饰位点,以对抗耐药性。进行了广泛的抗菌筛查以描绘全面的 SAR。许多衍生物在体外恢复了活性,对获得性或内在耐药性表现出 4-128 倍或 2-16 倍的增强,毒性较低。值得注意的是,最佳化合物 4g 表现出更好的药代动力学和药效学特征。进一步的研究揭示了 4g 的其他独立和协同机制,包括通过增加脂质 II 结合增强膜活性和增强对肽聚糖生物合成的抑制,突出了其作为未来补充糖肽抗生素库的主要候选者的潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号